The human immune system: a biological antivirus. Anti-virus database
I apologize for the unrealistically huge break after the first part: (
Link to the first part (innate immunity)
So, something that has entered the body has been recognized as hostile and destroyed. But each time, identifying extraneous entities by common non-specific signs is far from an optimal concept of behavior, since pathogens can develop a disguise system that will prevent their detection. In order to detect these microorganisms (and also to increase the effectiveness of the response with respect to all the others), a specific (she acquired) immune system has emerged, which includes T-lymphocytes and B-lymphocytes producing antibodies.
Formation and installation of anti-virus databases
')
T cells.
T-lymphocytes can recognize foreign agents using T-cell receptors (TCR - T cell receptor) - special protein molecules located on the cell surface. These receptors are not able to interact with extraneous proteins directly - they require that the protein fragment is presented attached to the main histocompatibility complex (MHC - major histocompatibility complex) already described in the previous part. Why such a strange decision at first glance has been worked out will be explained a little lower.
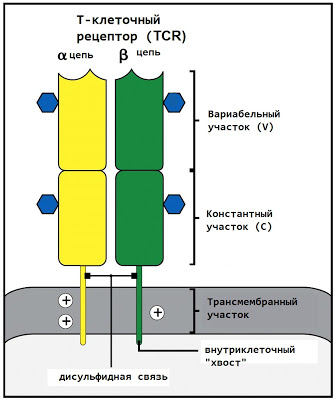
The T-cell receptor consists of two peptide chains (α and β), each of which is located at one end in the cytoplasm of the cell and is able to send signals into the cell, and the other (which will be discussed) in the intercellular space.
The outer end of each TCR chain consists of two fragments - variable (V) at the end and constant (.C) closer to the membrane. The constant region is the same in all human T lymphocytes, while the variable region may vary.
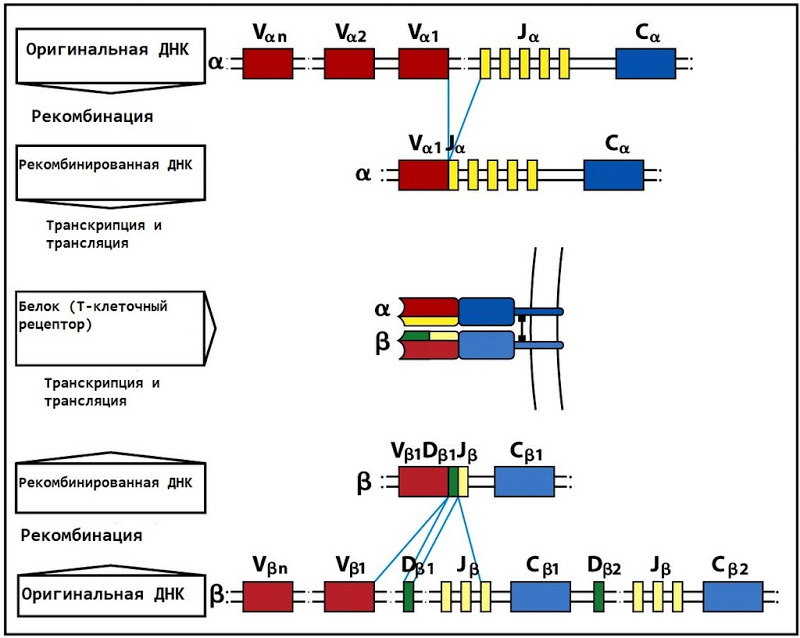
The variable region is encoded by two (for the α-chain - V and J) or three (for the β-chain - V, D and J) DNA segments. At the same time, there are more than 50 different V-fragments in the genome, 2 D and, on average, 40 (13 for α and 61 for β) J-fragments. When a T-cell forms in the bone marrow, the V, D, and J fragments are randomly selected from the existing heap. In addition, there are several more subtle mechanisms for introducing entropy, so that after all this the total number of possible TCRs is of the order of 10 ^ 18.
The T cells formed in this way leave the bone marrow and go to the thymus gland where they will be finely adjusted to existing antigens. At this stage, lymphocytes are not yet active, as randomly formed receptors can threaten surrounding tissues.
Fine-tuning in the thymus goes in several directions at once. The first one is positive selection. T cells try to interact with the antigen presenting cells (antigen-presenting cell - APC), on the surface of which are MHC molecules. If a T-cell fails to attach to the MHC, the cell will not divide. If it can - such a cell will give offspring.
The second is negative selection. In the thymus, proteins are produced from the whole body (including, for example, insulin), although in meager amounts - only in order to present them on the surface of the APC. If a T-cell recognizes such a protein, it is immediately destroyed; An error in the process of elimination usually leads to autoimmune diseases (in the case of insulin, to type 1 diabetes).

Now it should begin to become clear why T-cells are necessary for MHC. The process of the first contact of the T-cells with the antigen must be controlled, otherwise it may begin to pose a threat to the tissues of the body.
Thus, from a heap of randomly formed T-cells by positive and negative selection, a set of lymphocytes is obtained, which are able to recognize proteins attached to the MHC and safe for their own tissues. After that, these cells are sent to the lymph nodes, where they are activated.
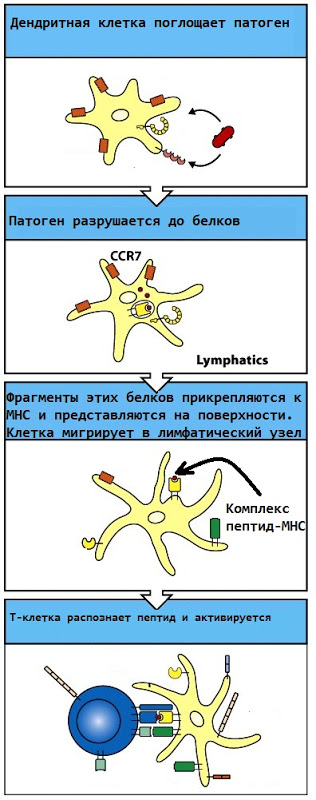
Activation of T-lymphocytes is a process after which these cells become able to actually perform their functions (destroy virus-infected cells or activate B-cells and macrophages). In the lymph node there is a huge number of dendritic cells, which, at the site of infection, absorb the infectious agent, break it down into proteins and present the corresponding proteins on their surface. After that, if the T-lymphocyte is able to recognize this protein (that is, its receptor fits under it), the T-lymphocyte “turns on”. Thus, only those lymphocytes that actually recognize the foreign protein are active.
After activation, T-lymphocytes are able to divide (clonal expansion), and freshly created copies of the lymphocyte are activated on the spot and enter the bloodstream or move to another area of the lymph node and there are responsible for the activation of B-cells.
B cells
Oddly enough, in general, they are quite similar to T lymphocytes. The primary means of recognition, the B-cell receptor, is inherently an antibody attached to the cell surface. So let's look at the structure of the antibody.
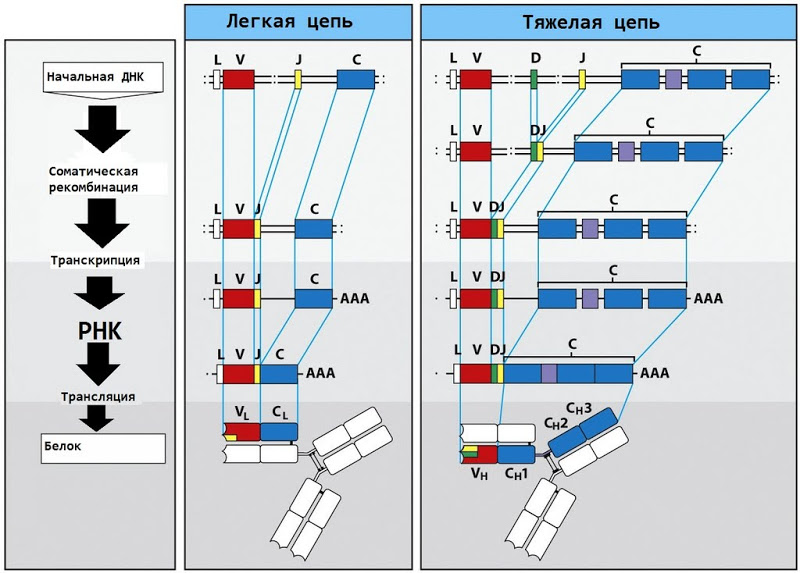
The basic IgM antibody (immunoglobulin M) consists of two heavy chains and two light chains; each light chain, like the T-cell receptor chains, consists of a variable and constant region.

The mechanism for generating the entropy of a variable region is again similar to the TCR mechanism. There are 40 possible V-fragments and 5 possible J-fragments, which, if you add to them a series of random mutations, produce a huge number of different possible antibodies.
The fundamental difference between T-and B-lymphocytes lies in the mechanism of selection and activation. B-cells do not undergo typical positive and negative selection. To avoid the threat of auto-activity, B-lymphocytes use T-cell selection.
After leaving the bone marrow, B-cells are sent straight to the lymph nodes. On the way, they can capture some proteins with their receptors, absorb them and present them on their surface attached to the MHC molecule.
To activate the B-cell, it is necessary to interact with the T-cell. In this interaction, the T-lymphocyte recognizes the MHC-protein complex on the surface of the B-lymphocyte and, if recognition has occurred, activates the B-lymphocyte. As it is not difficult to notice, ineffective or autoreactive B-cells simply will not be activated, as T-lymphocytes that could do this were removed during negative selection or were not activated.

After activation, the B-cell goes through a process called somatic hypermutation. It begins to divide frequently, and with each division, all new mutations are introduced into the DNA region that encodes the antibody variable region. Obviously, B-cells that better recognize the antigen will be more frequently and actively activated, and we get ... That's right, normal natural selection. Which eventually creates B-cells, the antibodies of which perfectly recognize some kind of antigen.
Ultimately, the B lymphocyte becomes a plasma cell that begins to produce antibodies on an industrial scale, and after the threat has passed, it becomes a memory cell that can produce suitable antibodies after a considerable period of time.
Thus, the generation of “anti-virus databases” of the human body occurs randomly, however, only those signatures that have shown their effectiveness on pathogens that have already been recognized by heuristic mechanisms become active and actually used.
The "Friend-Alien" system. MHC role
Unlike macrophages - professional phagocytes that simply devour pathogens, lymphocytes are not capable of this, this is not their function. B cells exert their influence by secreting antibodies, and T cells by interacting with other cells.
All T-cells can be divided into three types: T-killers, T-helpers and T-suppressors. About T-suppressors I will try to tell in one of the following parts.
T-killers are designed to kill pathogens (viruses or bacteria) that are inside cells. For these purposes, in addition to the TCR, they carry nearby CD8 molecules on the surface, and together the TCR and CD8 recognize type I MHC molecules.
MHC I are produced on the surface of every cell in the body. In their absence, the cell is killed by a natural killer (NK cell). All proteins that are inside the cell are attached to MHC I. T-killer does not respond to any of them (due to negative selection). However, if the pathogen is inside the cell, it will also be presented on the surface and will most likely be recognized by the T-killer, and the cell, along with the filth inside, will be destroyed.
MHC II is another case. They are found only on the surface of APC - antigen-presenting cells (these are macrophages, dendritic cells and B-cells), and their T-helper cells are recognized by means of CD4 molecules similar to CD8.
If proteins that are in the cytoplasm of a cell are attached to MHC I, then proteins that have been absorbed by the cell from the outside are attached to MHC II. Thus, proteins on the surface of the APC - this is a kind of board "they are wanted." T-helpers are first activated themselves after contact with a dendritic cell, and then they activate B-cells (which begin to produce antibodies) and macrophages (which begin to devour bacteria more actively).

The resulting system is able to recognize the majority of infectious agents, both those that live between cells and intracellular ones. In addition, it does not waste extra forces on copying obviously ineffective cells and does not attack its own tissue.
Thank you very much for your attention and unfathomable respect to those who could read this heavy text to the end :)
Link to the first part (innate immunity)
So, something that has entered the body has been recognized as hostile and destroyed. But each time, identifying extraneous entities by common non-specific signs is far from an optimal concept of behavior, since pathogens can develop a disguise system that will prevent their detection. In order to detect these microorganisms (and also to increase the effectiveness of the response with respect to all the others), a specific (she acquired) immune system has emerged, which includes T-lymphocytes and B-lymphocytes producing antibodies.
Formation and installation of anti-virus databases
')
T cells.
T-lymphocytes can recognize foreign agents using T-cell receptors (TCR - T cell receptor) - special protein molecules located on the cell surface. These receptors are not able to interact with extraneous proteins directly - they require that the protein fragment is presented attached to the main histocompatibility complex (MHC - major histocompatibility complex) already described in the previous part. Why such a strange decision at first glance has been worked out will be explained a little lower.
The T-cell receptor consists of two peptide chains (α and β), each of which is located at one end in the cytoplasm of the cell and is able to send signals into the cell, and the other (which will be discussed) in the intercellular space.
The outer end of each TCR chain consists of two fragments - variable (V) at the end and constant (.C) closer to the membrane. The constant region is the same in all human T lymphocytes, while the variable region may vary.

The variable region is encoded by two (for the α-chain - V and J) or three (for the β-chain - V, D and J) DNA segments. At the same time, there are more than 50 different V-fragments in the genome, 2 D and, on average, 40 (13 for α and 61 for β) J-fragments. When a T-cell forms in the bone marrow, the V, D, and J fragments are randomly selected from the existing heap. In addition, there are several more subtle mechanisms for introducing entropy, so that after all this the total number of possible TCRs is of the order of 10 ^ 18.

The T cells formed in this way leave the bone marrow and go to the thymus gland where they will be finely adjusted to existing antigens. At this stage, lymphocytes are not yet active, as randomly formed receptors can threaten surrounding tissues.
Fine-tuning in the thymus goes in several directions at once. The first one is positive selection. T cells try to interact with the antigen presenting cells (antigen-presenting cell - APC), on the surface of which are MHC molecules. If a T-cell fails to attach to the MHC, the cell will not divide. If it can - such a cell will give offspring.
The second is negative selection. In the thymus, proteins are produced from the whole body (including, for example, insulin), although in meager amounts - only in order to present them on the surface of the APC. If a T-cell recognizes such a protein, it is immediately destroyed; An error in the process of elimination usually leads to autoimmune diseases (in the case of insulin, to type 1 diabetes).

Now it should begin to become clear why T-cells are necessary for MHC. The process of the first contact of the T-cells with the antigen must be controlled, otherwise it may begin to pose a threat to the tissues of the body.
Thus, from a heap of randomly formed T-cells by positive and negative selection, a set of lymphocytes is obtained, which are able to recognize proteins attached to the MHC and safe for their own tissues. After that, these cells are sent to the lymph nodes, where they are activated.
Activation of T-lymphocytes is a process after which these cells become able to actually perform their functions (destroy virus-infected cells or activate B-cells and macrophages). In the lymph node there is a huge number of dendritic cells, which, at the site of infection, absorb the infectious agent, break it down into proteins and present the corresponding proteins on their surface. After that, if the T-lymphocyte is able to recognize this protein (that is, its receptor fits under it), the T-lymphocyte “turns on”. Thus, only those lymphocytes that actually recognize the foreign protein are active.

After activation, T-lymphocytes are able to divide (clonal expansion), and freshly created copies of the lymphocyte are activated on the spot and enter the bloodstream or move to another area of the lymph node and there are responsible for the activation of B-cells.
B cells
Oddly enough, in general, they are quite similar to T lymphocytes. The primary means of recognition, the B-cell receptor, is inherently an antibody attached to the cell surface. So let's look at the structure of the antibody.
The basic IgM antibody (immunoglobulin M) consists of two heavy chains and two light chains; each light chain, like the T-cell receptor chains, consists of a variable and constant region.

The mechanism for generating the entropy of a variable region is again similar to the TCR mechanism. There are 40 possible V-fragments and 5 possible J-fragments, which, if you add to them a series of random mutations, produce a huge number of different possible antibodies.

The fundamental difference between T-and B-lymphocytes lies in the mechanism of selection and activation. B-cells do not undergo typical positive and negative selection. To avoid the threat of auto-activity, B-lymphocytes use T-cell selection.
After leaving the bone marrow, B-cells are sent straight to the lymph nodes. On the way, they can capture some proteins with their receptors, absorb them and present them on their surface attached to the MHC molecule.
To activate the B-cell, it is necessary to interact with the T-cell. In this interaction, the T-lymphocyte recognizes the MHC-protein complex on the surface of the B-lymphocyte and, if recognition has occurred, activates the B-lymphocyte. As it is not difficult to notice, ineffective or autoreactive B-cells simply will not be activated, as T-lymphocytes that could do this were removed during negative selection or were not activated.

After activation, the B-cell goes through a process called somatic hypermutation. It begins to divide frequently, and with each division, all new mutations are introduced into the DNA region that encodes the antibody variable region. Obviously, B-cells that better recognize the antigen will be more frequently and actively activated, and we get ... That's right, normal natural selection. Which eventually creates B-cells, the antibodies of which perfectly recognize some kind of antigen.
Ultimately, the B lymphocyte becomes a plasma cell that begins to produce antibodies on an industrial scale, and after the threat has passed, it becomes a memory cell that can produce suitable antibodies after a considerable period of time.
Thus, the generation of “anti-virus databases” of the human body occurs randomly, however, only those signatures that have shown their effectiveness on pathogens that have already been recognized by heuristic mechanisms become active and actually used.
The "Friend-Alien" system. MHC role
Unlike macrophages - professional phagocytes that simply devour pathogens, lymphocytes are not capable of this, this is not their function. B cells exert their influence by secreting antibodies, and T cells by interacting with other cells.
All T-cells can be divided into three types: T-killers, T-helpers and T-suppressors. About T-suppressors I will try to tell in one of the following parts.
T-killers are designed to kill pathogens (viruses or bacteria) that are inside cells. For these purposes, in addition to the TCR, they carry nearby CD8 molecules on the surface, and together the TCR and CD8 recognize type I MHC molecules.
MHC I are produced on the surface of every cell in the body. In their absence, the cell is killed by a natural killer (NK cell). All proteins that are inside the cell are attached to MHC I. T-killer does not respond to any of them (due to negative selection). However, if the pathogen is inside the cell, it will also be presented on the surface and will most likely be recognized by the T-killer, and the cell, along with the filth inside, will be destroyed.
MHC II is another case. They are found only on the surface of APC - antigen-presenting cells (these are macrophages, dendritic cells and B-cells), and their T-helper cells are recognized by means of CD4 molecules similar to CD8.
If proteins that are in the cytoplasm of a cell are attached to MHC I, then proteins that have been absorbed by the cell from the outside are attached to MHC II. Thus, proteins on the surface of the APC - this is a kind of board "they are wanted." T-helpers are first activated themselves after contact with a dendritic cell, and then they activate B-cells (which begin to produce antibodies) and macrophages (which begin to devour bacteria more actively).

The resulting system is able to recognize the majority of infectious agents, both those that live between cells and intracellular ones. In addition, it does not waste extra forces on copying obviously ineffective cells and does not attack its own tissue.
Thank you very much for your attention and unfathomable respect to those who could read this heavy text to the end :)
Source: https://habr.com/ru/post/85634/
All Articles