Bubble Physics: Search for a Foam Breakdown Mechanism

The simplest things can have the most unusual and even unexplored aspects. From an early age we try to understand the nature of everything that surrounds us. How the light bulb works in the chandelier, why the sky is blue, where the rain comes from, why the lemon is sour, and the sugar is sweet - this is just a small list of questions that can be asked by a curious child in a very short period of time. Growing up, we are not so much interested in such things, paying attention to something more important, in our opinion. But understanding the nature of simple, at first glance, things can be of great benefit.
Today we will get acquainted with a very unusual research in which scientists tried to understand the mechanism of foam destruction. Have you ever wondered why the foam in your cappuccino is not as durable as you would like? If you were told that you simply do not know how to prepare it, then now you will have a very scientific counter argument. What kind of a series of events leads to the destruction of the structure of the foam, what is the catalyst for this process and what is the use of such knowledge? We will find answers to these and other questions in the report of the research group. Go.
')
The basis of the study
No matter how simple the foam seems at first glance, it still remains a complex system with a gas-dispersed phase and a liquid / solid dispersion medium. If we talk about the most common foam consisting of gas bubbles and liquid films, then such a structure is considered to be a non-equilibrium system. Also, the foam can be called a polydisperse system due to the fact that the composite bubbles can be completely different sizes. In addition, the foam is very unstable and from that very short-lived system due to the fact that the density of a liquid is hundreds or even thousands of times greater than the density of a gas.
Despite this, the foam is quite common in human life and used in various fields. They are present in everyday life (whipped cream, shaving foam, etc.), in biotechnology (foam in bioreactors), in chemical technology (froth flotation), and even in pharmacology. If we study the mechanism behind the process of foam destruction, then we can make it more durable, as the researchers themselves say.
In their work, they point to three main processes governing the dynamics of foam: consolidation, drainage and destruction. Enlargement is the process of reducing the number of bubbles, but increasing their size, which is caused by different pressures between the bubbles. Drainage is the process of thinning films, that is, the walls of bubbles, due to the flow of water under the influence of gravity.
These two stages of foam life have previously been sufficiently well studied, as, in general, and the process of destruction. Previous studies have shown that bubble collapse occurs when the lower limit of coalescence * in a volume fraction of liquid is reached.
Coalescence * - the fusion of particles inside the mobile environment (for example, bubbles in the foam).The relationship between the destruction of the bubble and the rearrangement of T1 * was also established by introducing additional air into the system.
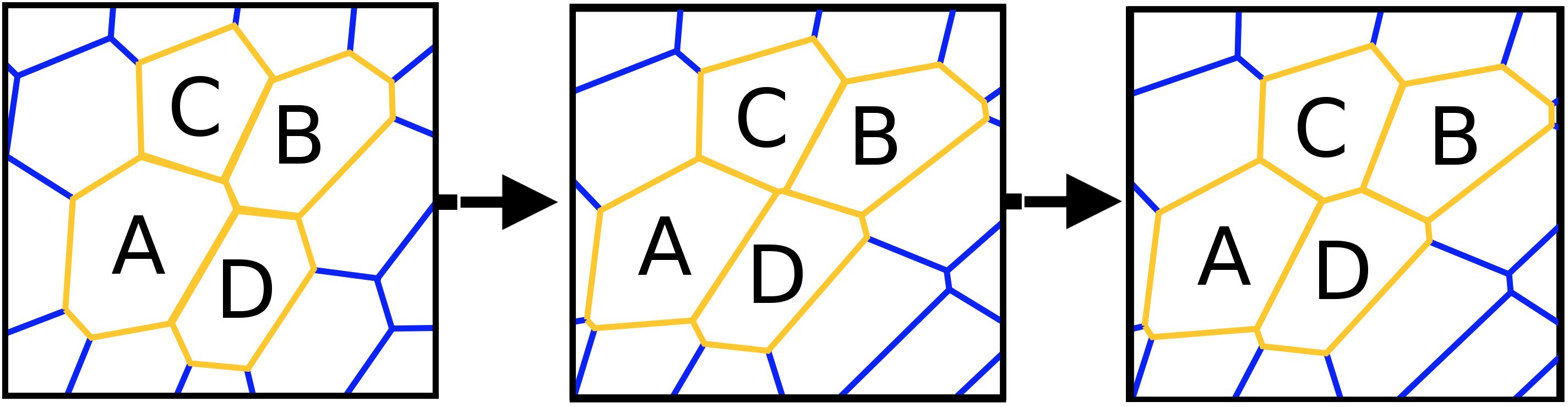
The process T1 * or the rearrangement T1 * - the process of changing the shape of cellular materials (foam, biological tissue, etc.), consisting of droplets, bubbles, cells.At the time of the destruction of the bubble could hear a light "cotton" (the release of gas). By measuring and analyzing the acoustic data at the time of the destruction of the foam, the scientists came to the conclusion that this process arises due to the collective collapse of bubbles (CCP), in other words, cascade collapse.
At the beginning of the process there are 4 objects (A, B, C and D). A and B are in contact, C and D are located on both sides of AB, that is, they are not in contact with each other. Breaking the connection between A and B with the subsequent establishment of a connection between C and D is the process T1.
But KKP is only the tip of the iceberg, and the mechanism that launches it into action is unknown. This is how to understand that the chain of dominoes is destroyed, because the dominoes fall on each other, but do not know which of them was the first and that it overturned (a rough, but understandable allegory).
In this work, scientists used a quasi-two-dimensional foam as an “experimental” one, on the edge of which 1 vial burst. Scientists observed the destruction of the entire foam cascade using a high-speed camera, after which they analyzed the records. Two mechanisms of collapse propagation were established: propagation and penetration.
As φ (volume fraction) increases, it becomes more and more difficult for liquid droplets to penetrate liquid films, which causes drops to rebound from the films due to their elasticity or absorption of the droplets by the film. More on this in the results of observations.
Research results
The KKP process (collective collapse of bubbles) was observed at different values of φ . So, for example, image 1a shows the PSC from time t = 0 ms to 3.12 ms for φ = 0.0099.
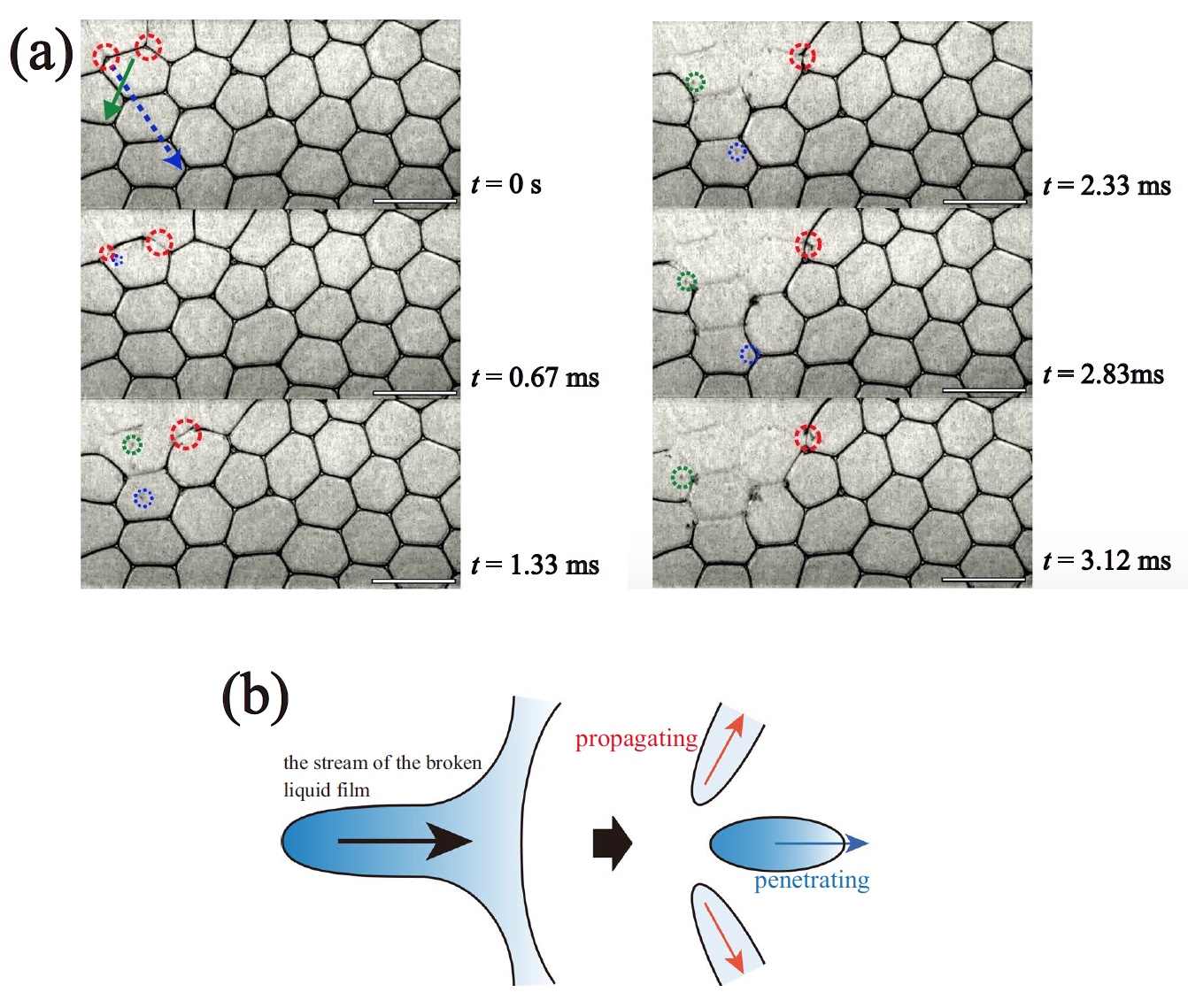
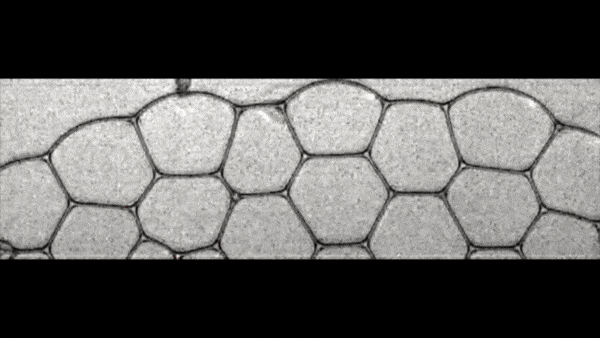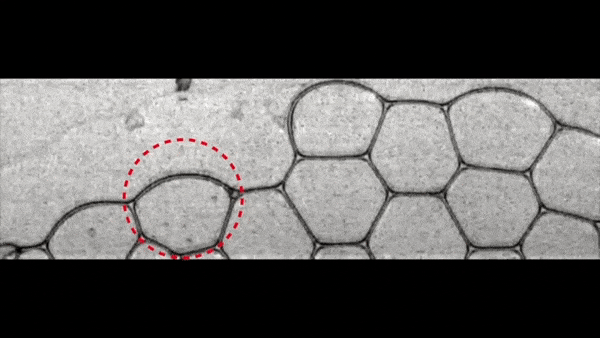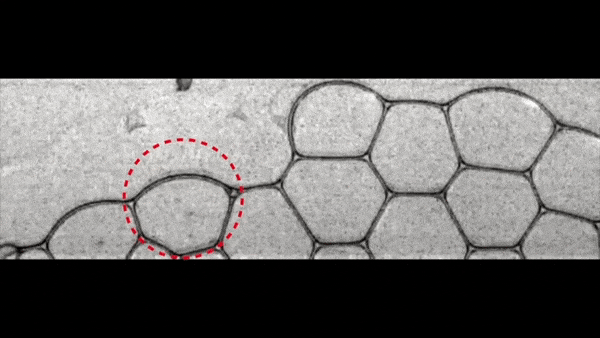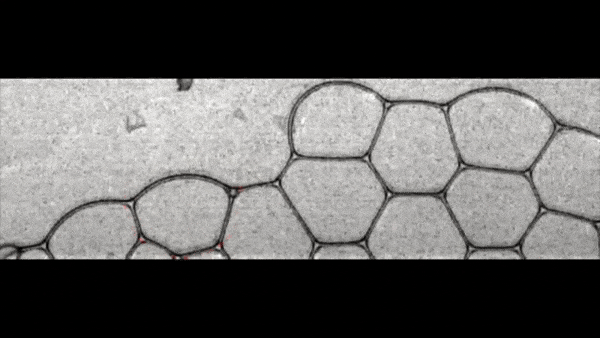
Image number 1
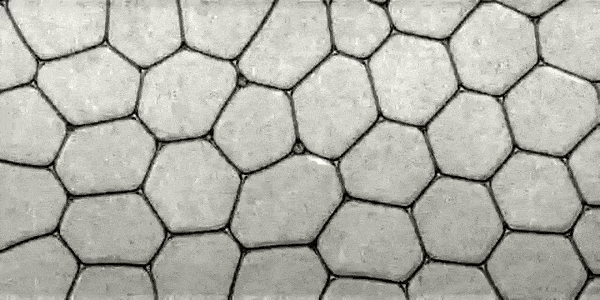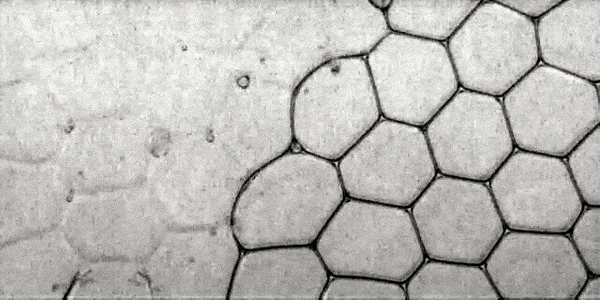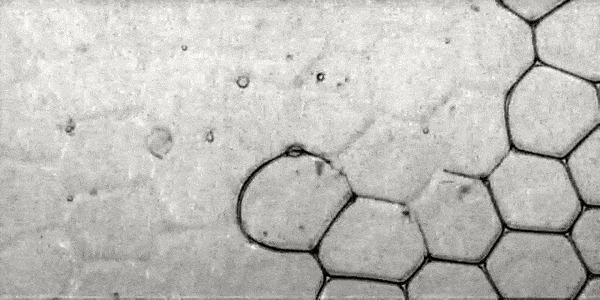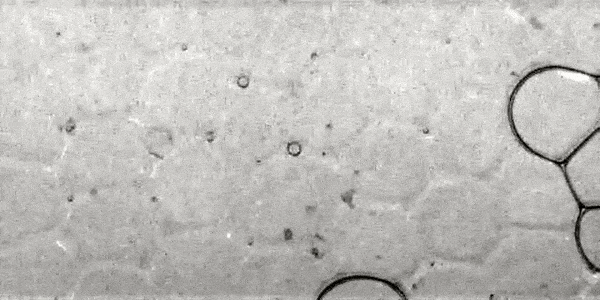
KKP process ( t = 0 ... 3.12 ms, φ = 0.0099).
A capillary glass needle was used to puncture the vial. The collapse of bubbles at the beginning of the QPC process is observed along the outer edges of the foam, which the scientists decided to call the surface effect. Behind this, two CCP processes begin inside the foam itself, so to speak in its total volume: propagation and penetration.
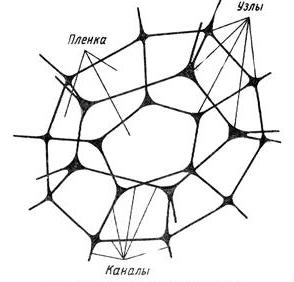
When a liquid film is torn, it is quickly absorbed by the Plateau Channel.
According to the Plateau law, the ribs of a bubble in a foam are channels filled with a dispersive medium. In the same channel can converge only three films located at angles of 120 °.Due to the strong absorbing effect, the next liquid film converging in the same Plato channel is also broken (red circle by 1a ). This sequential (cascading) process of destruction of foam bubbles is one of the PSC processes - propagation ( 1b ).
Cell structure (bubble) foam.
At the same time, at the moment of absorption of the burst film by the Plateau channel, a drop of liquid is released (blue and green circles at 1a ). The droplets fall inside the foam into the film removed by the liquid (arrows at 1a ). The velocity of these droplets (V d ) was about 3 m / s. This FCC process, referred to as penetration, results in the destruction of the removed films ( 1b ).
Full collapse occurs with multifaceted destruction of the film bubbles through both variants of the CCP.
If, however, φ is increased to ≥ 0.015, the probability of a liquid drop appearing at the time of the destruction of the bubble film is greatly reduced. And the speed of the still-occurring drops also becomes smaller, making it more difficult for drops to penetrate the removed films. Instead of penetration, a drop rebounds.
Bouncing drops (instead of penetration) at φ ≥ 0.015.
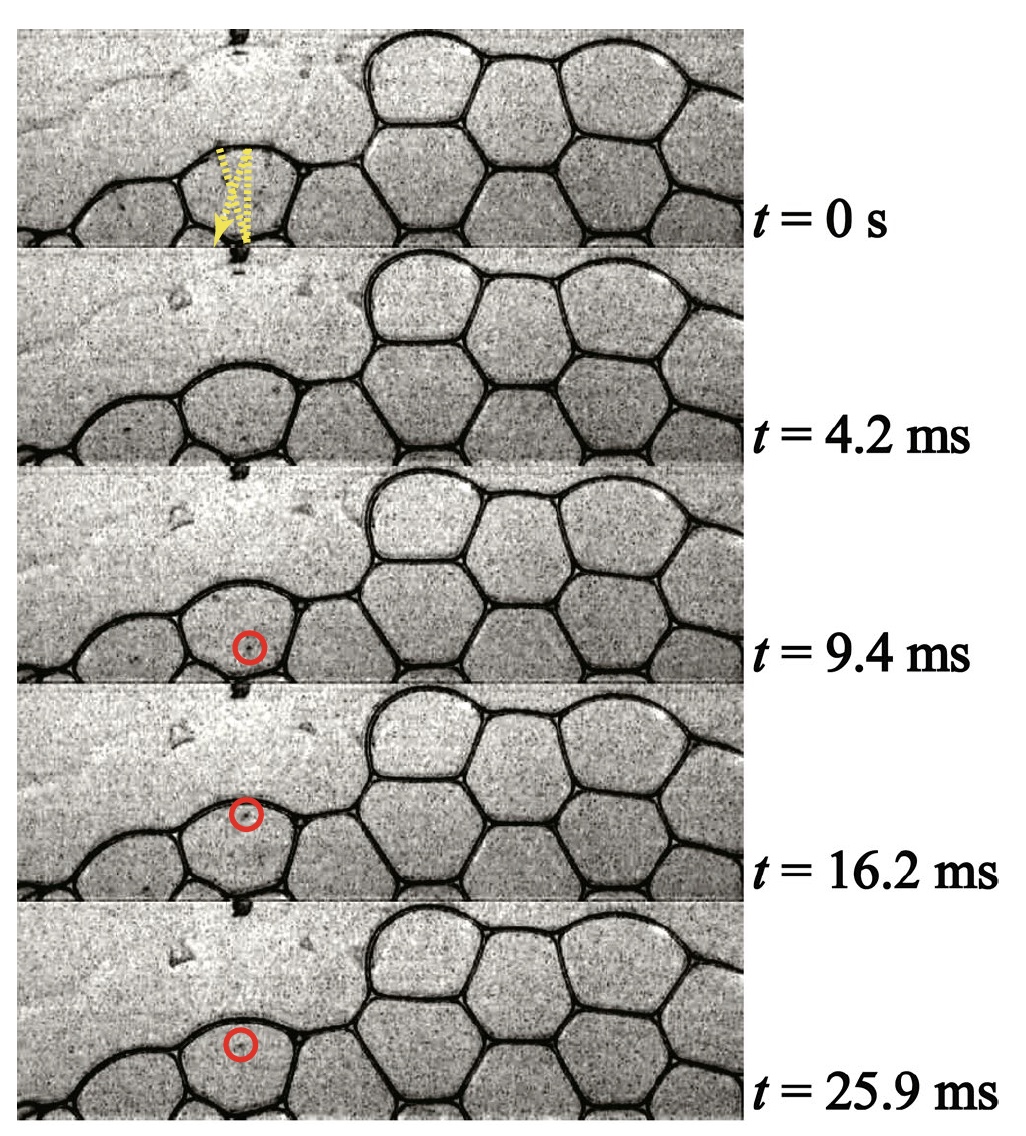
Image number 2
The image above shows how the drop bounces off the films for 30 ms (the dotted line is the trajectory of the drop).
Measuring the velocity of the drop (V d ) after each rebound, you can construct a graph of V d versus the number of impacts (n i ).
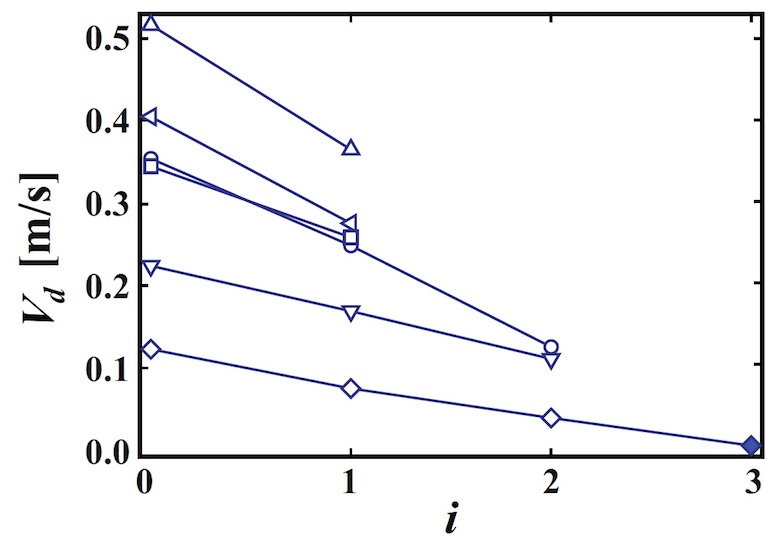
Image number 3: the dependence of the speed of the number of rebounds.
As expected, the drop rate decreases with increasing number of bounces. In this case, you can determine the recovery coefficient of the film as e = | V d (i + 1) | / | V d (i) |, where | V d (i) | - drop rate after the i-th rebound. Using observational data, it was found that e = 0.50 ~ 0.74. After the i-th rebound, a drop is absorbed by a liquid film.
With a further increase in φ (> 0.022), the film is successfully absorbed by the Plato channel, but liquid drops do not occur. Bubbles along the edge of the foam burst from the surface effect and at higher rates of φ , but the number of such bubbles is greatly reduced, and the process of collapse quickly stops. In other words, the PSC process does not arise.
Next, scientists investigated the dependence of the number of bursting bubbles on the indicator φ. The set of collapsing bubbles (N total ) consists of those that burst on the edge of the foam due to the surface effect, and those that are destroyed due to penetration and propagation.
Also in the calculations, the indicator N inner is used - the number of collapsing bubbles in the foam volume minus the outer edges. Counting collapsing bubbles was carried out from the first to the last bubble, which took about 0.04 seconds.
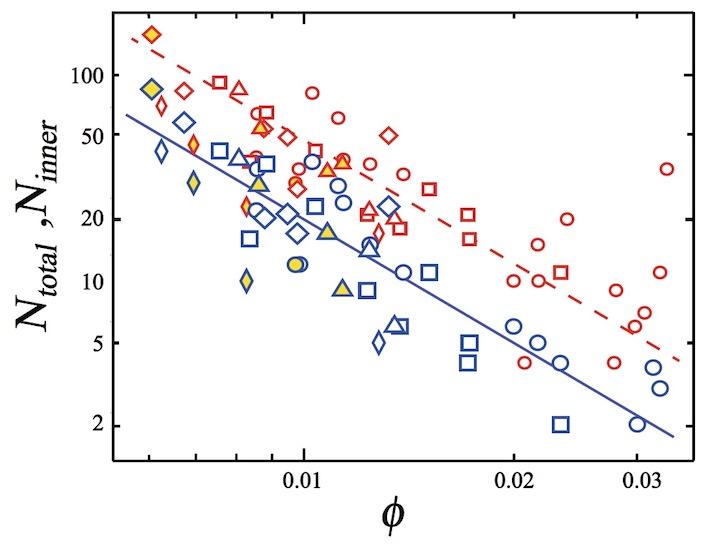
Image number 4
The image above shows the N total (red) and N inner (blue) values relative to φ . Triangles, circles and squares correspond to N total or N inner at N f ~ 200 for glycerol concentrations of 9.4%, 17.8% and 29%, respectively (N f is the total number of bubbles in the foam).
As we see from the graph, the value of N total and N inner decreases with increasing φ . Applying the power law * , scientists established that N inner ∝ φ −γ e with γ e = 2.3 ± 0.36.
The power law * is a functional dependence of two quantities, when a change in one leads to a proportional change in the other.It was also found that the indicators N total and N inner do not depend on the concentration of glycerol, if it is below 29%. If the concentration increases to 40%, then piercing the vial becomes more difficult, and the PSC process does not occur.
The study of collapsing bubbles in the case of a larger foam (N f ~ 500) showed that their number does not depend on the total number of bubbles (diamonds on the graph above), that is, N total and N inner do not depend on N f .
As we remember, a glass needle was used for the puncture. It was coated with silicone grease to improve piercing. Scientists checked how this affects the value of N total and N inner , having made punctures without lubrication. Thus, the PSC process arose spontaneously. However, as was to be expected, the use of the lubricant had no effect on the number of collapsing bubbles and on the CCP process as a whole.
If φ is small, then the shape of each bubble is anisotropic, and the distorted bubbles form a kind of chain. Bubbles of anisotropic shape and / or large size have a large excess surface energy, therefore, they are more easily destroyed.
Given this, the scientists decided to check the relationship between the CCP and the shape of the bubbles. For this, the parameter λ i was used as a characteristic of the anisotropy of bubble i . λ i is determined by the following formula:
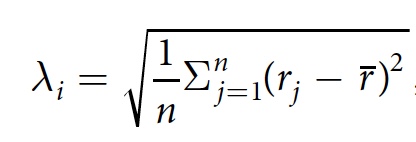
where j is the pixel on the edge of the bubble, n is the total number of pixels j , r j is the distance between the center of the bubble i and pixel j , r is the average distance r j .
λ i will be equal to 0 if the bubble i is round. If it is anisotropic, then λ i > 0.
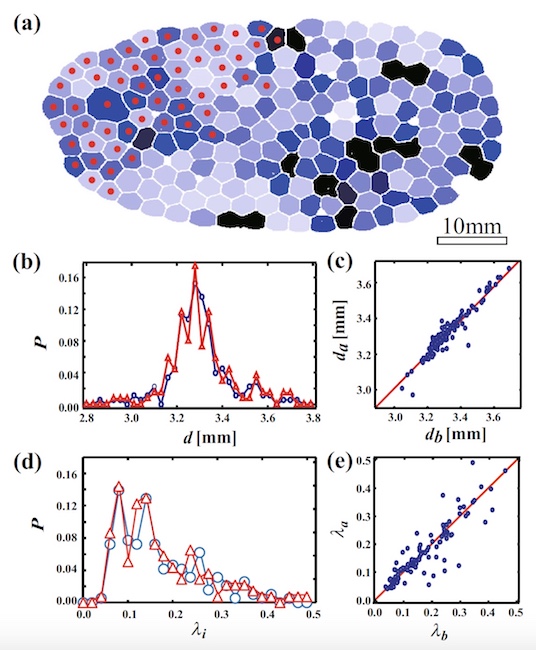
Image number 5
Image 5a shows foam at φ = 0.0086 prior to the start of the QPC process. The bubbles are colored from black (λ i more) to white (λ i less). Red dots indicate that the bubbles collapsed during the PAC.
Scientists have discovered that the bubbles on the left are evenly collapsed. Graph 5b shows the probability distribution as a function of the average bubble diameter i (d i ) before and after the BCP of all bubbles. The diameter (d i ) was calculated by averaging the distance between the center and the interface of the bubble. As we can see, the form of the probability distribution on the graph after the CCP is the same as before the CCP.
On the graph 5c, the ratio of the diameter after the CCP (d a ) and before the CCP (d b ). Obviously, d a = d b , that is, the diameter of the bubbles did not change during the cascade collapse.
Plot 5d shows λ i (bubble anisotropy characteristic) before and after collapse. This indicator also did not change, despite the cascade collapse (λ a (before CCP) = λ b (after CCP); 5e ).
All these observations suggest that the shape of the bubbles does not change due to the cascade destruction of the foam, and that the shape does not affect this process.
The next probable factor affecting the process of foam destruction is the mechanical effect of a broken bubble film on its neighbors. To determine the effect of this factor, scientists measured the rate of film breakage at a glycerin concentration of 17.8% using the formula V = l / t , where l is the film length and t is the time required for film absorption from beginning to end.
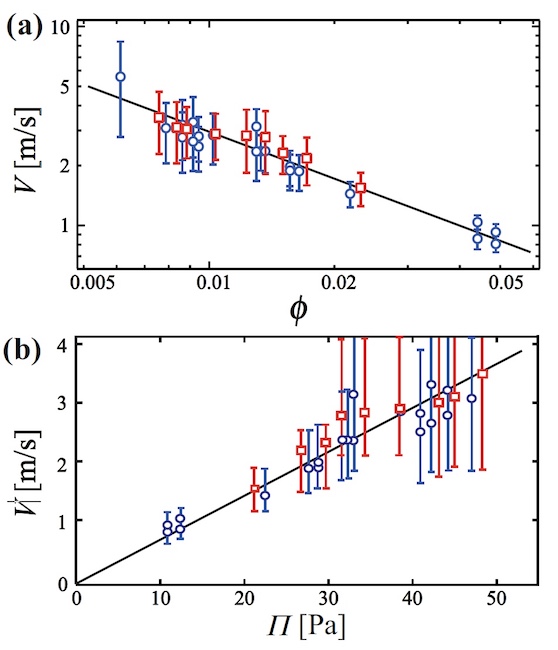
Image number 6
Graph 6a shows the dependence of V on φ in the form of a logarithmic graph. Calculations show that V ~ 10 m / s with a glycerol concentration of 17.8% (circles on the graph). In the case of glycerol concentration of 29%, the rate remained almost unchanged (squares on the graph).
As φ increases, the speed decreases, due to which the films formed during the break bounce off other channels and, as a result, are absorbed by them.
The relationship between speed and osmotic pressure has also been studied ( 6b ).
The pressure formula for a two-dimensional foam is as follows:
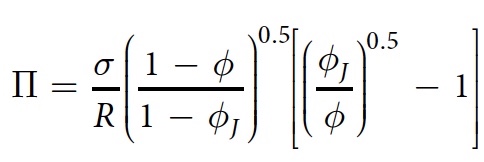
where σ is the surface tension, R is the average bubble radius, φ J is the jamming point, equal to 0.16 in two dimensions.
In their work, scientists used the following indicators: σ = 37 mN / m and R = 1.7 mm.
If we assume that the thickness of the film layer is 1 μm, then a proportional dependence of V on Π ( 6b ) is visible. Therefore, the driving force of absorption is the negative pressure in the film.
Finally, scientists conducted an analysis of the ratio of N inner and speed V (image below).
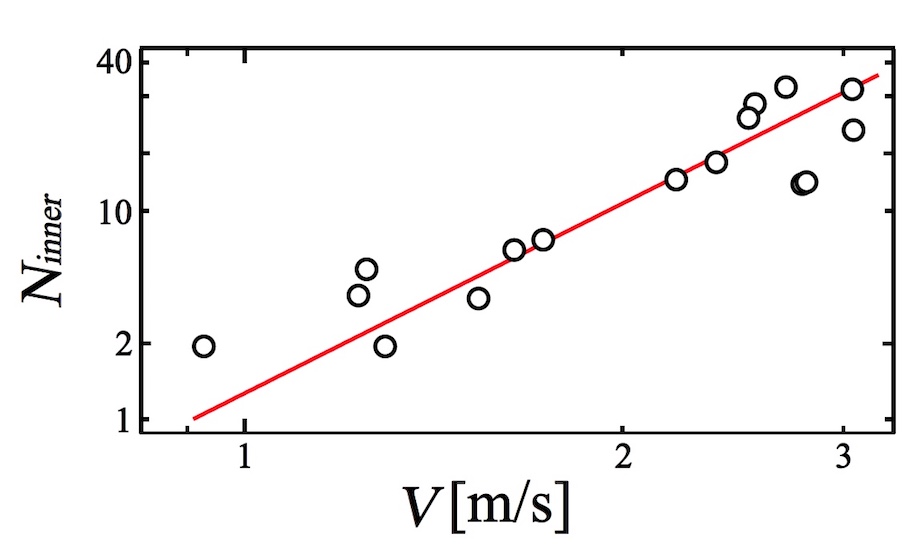
Image number 7
Scientists have found that the N inner index greatly increases with increasing film flow rate. Thus, we can conclude that the process of penetration is a crucial element in the process of the general collapse of the foam.
For a more detailed acquaintance with the nuances of the study I recommend to look into the report of scientists .
Epilogue
In this work, scientists were able to find out that at the time of the destruction of the foam, two processes play a major role - absorption and propagation. In addition, scientists have found that the increase in the proportion of liquid velocity drops, resulting from the destruction of the bubble film, is reduced. Therefore, it is more difficult to destroy all the foam. Instead of absorbing a drop by another distant film, a multiple rebound of the drop occurs, and only then absorption.
Scientists intend to continue to study the foam, in order to understand its strengths and weaknesses. In their opinion, this work will improve the foam, making it more durable and sustainable. And such advantages can be useful both in everyday life and in laboratories engaged in the production and study of various biological and chemical substances, materials and other things.
Who would have thought that in the 21st century scientists would actually study beer foam, looking for ways to make it stronger. But, strange as it may sound, any knowledge is important, any knowledge is needed. Understanding the world and everything that fills it, allows us to better use what was invented or discovered long ago, or improve it in accordance with the constantly changing conditions of our life.
Thank you for your attention, stay curious and have a good working week, guys! :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to friends, 30% discount for Habr's users on a unique analogue of the entry-level servers that we invented for you: The Truth About VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps $ 20 or how to share the server? (Options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to build an infrastructure building. class c using servers Dell R730xd E5-2650 v4 worth 9000 euros for a penny?
Source: https://habr.com/ru/post/455533/
All Articles