Fresh air on Mars: bend a CO2 molecule and get oxygen

What do we remember in the phrase "science fiction"? Of course, robots, flying machines, a study of previously unexplored corners of the universe, aliens, and even a whole series of plots. Among them, a special place has always been occupied by the topic of settling another planet, whether from confidence in the inevitability of their own death, or from the desire to radically change the situation. The first contender for the title Earth 2.0 has always been Mars, cold and lifeless. At the moment, it is not possible to pack your bags and fly to Mars on vacation for two weeks, but this is absolutely real and doable, the only question is how soon. But the most conspicuous minus of Mars (except for the absence of a normal Internet) is the absence of an atmosphere acceptable to life. Fly to your destination, get out of the aircraft and breathe deeply, feeling all the freshness of the local air - this is not about Mars. But this will not always be the case.
Today we will get acquainted with the study, which describes a new method of oxygen generation through an unusual chemical reaction. How did scientists produce molecular oxygen from CO 2 , how effective is this method, and where can it be used other than interplanetary travel? We will look for answers to these questions in the scientists' report. Go.
')
The basis of the study
A person needs only three things to live: food, water and air, which will have enough oxygen. These are our primary needs (yes, the Internet is not in this list). But oxygen just does not roll on the road, especially outside our beloved planet. Therefore, if we want to go somewhere, we need to take it with us and use various devices to clean the air and reuse it. All this joy takes a lot of space, and sooner or later oxygen will be exhausted. And to replenish stocks in space or on Mars will be, to put it mildly, problematic.
However, relatively recently, scientists have discovered a sufficiently large amount of oxygen in comet 67P / CG6, which prompted them to ask an obvious question: where did it come from? Oxygen in such bodies as a comet is the result of an abiotic reaction occurring under extreme conditions, during which from H 2 O, CO 2 , CO, etc. O 2 (oxygen) is released. Scientists say that such reactions can explain the presence of oxygen in comets, the upper atmosphere of Mars and in the early atmosphere of the Earth. A person can apply such a chemical reaction to produce O 2 from CO 2 , which will make Mars suitable for life.
If it is very exaggerated and briefly, then the above reaction is the decomposition of CO 2 into components: C + O 2 . In other words, dissociation.
The dissociation of CO 2 can take place under several scenarios, depending on the energy available for the reaction. Partial dissociation of CO 2 → CO + O (5.43 or 7.56 eV) requires the least energy. There is also a complete dissociation of CO 2 → C + O + O, requiring 16.46 eV. And, the most curious, exotic dissociation, when CO 2 decomposes into C and O 2 . Calculations show that a similar reaction takes place on the surface of the potential energy of the ground state, first forming a cyclic intermediate CO 2 compound [c – CO 2 ( 1 A 1 )], which is then reconstructed into a collinear intermediate COO ( 1 Σ + ) on the way to dissociation in C + O 2 .
Such a reaction is possible if one bends the molecule in such a way that the two O atoms become as close as possible to each other. It requires 6 eV of internal energy. And, you see, no matter how strong the metal bars are, it will be much more difficult to bend the molecule.
The transitions to electronically excited and anionic CO 2 states can help to bend the molecule. Scientists remind that recent experiments of their colleagues showed that the use of VUV (vacuum ultraviolet) photoexcitation and attachment of electrons makes it possible to achieve the dissociation of CO 2 into C ( 3 P) + O 2 (X 3 Σ g -). However, no one has previously studied this exotic process at a sufficiently detailed level. And all because as a result of such experiments no ionized O 2 products were detected. But, as we know, not finding something else does not mean that it does not exist at all.
Therefore, in the study we are considering today, scientists applied ion beam scattering methods along with mathematical modeling to demonstrate a new way to activate direct reduction of CO 2 to O 2 with the detection of ionized O 2 products. This process contains a previously unknown intramolecular reaction pathway that occurs during active collisions of ions and the surface of CO 2 . The most surprising is the lack of dependence of this reaction on the nature and temperature of the surface.
Research results
First of all, scientists demonstrate the formation of O 2 in hyperthermic CO 2 + / Au collisions by plotting the distribution of the kinetic energy of three scattered molecular ion products (CO 2 + , O 2 + and O 2 - ) for different incident CO 2 + energies (E 0 ) . At E 0 <80 eV, a very weak signal of scattered CO 2 + was detected ( 1a , graph on the left).

Image number 1
The peak energy of the released CO 2 + is proportional to E 0 , that is, there is a ballistic or pulsed rebound from the surface, which eliminates physical spraying. Scientists believe that observing the “dynamic” signal of CO 2 + is extremely important, because it is proof that a certain amount of CO 2 survives in a superficial collision. In addition, you can determine the sequence of collisions of constituent atoms. In addition to CO 2 + , signals of scattered O 2 ions were also observed ( 1b , 1c / graph in the center and to the right). In this case, the energies of the outgoing O 2 + and O 2 - represent a larger share of the energy of the incident particles (57%) and evenly increase parallel to E 0 in a wider range than the scattered CO 2 + . The maximum signal of O 2 ions was observed at E 0 ~ 100 eV.
The researchers called the detection of ionic O 2 products surprising, since neither the sputtering of surface O 2 nor the abstraction reaction of the O atom can explain the formation of these ions. And all because both of these mechanisms would produce O 2 at much lower output energies than was observed. It is logical to assume that the very dissociation of CO 2 is involved.
Both partial and complete dissociation of CO 2 is in good agreement with other detected ionic products (CO + , CO - , O + , O - and C + ). And the energy of the outgoing CO + , CO - and O - varies in parallel with the energy of the incident particles, which is consistent with the dynamic formation during surface collisions.
But the O + and C + peaks show an extremely small dependence on E 0 , which indicates a different origin, that is, sputtering. Confirmation of complete dissociation are scattered C + products, which manifest themselves at E 0 > 80 eV.
Next, scientists used kinematics to describe the mechanism of scattering.
The binary theory of collisions (BTS) makes it possible to calculate the kinematic factor, defined as the fraction of the energy of the incident particles held by the scattered product leaving the surface. In the case of the simplest model, CO 2 + is scattered as a whole molecule, i.e. like a solid sphere with an atomic mass of 44 Da. In this case, the BTS predicts a kinematic coefficient of 0.6349, which is rather weakly correlated with the data ( 2a ).
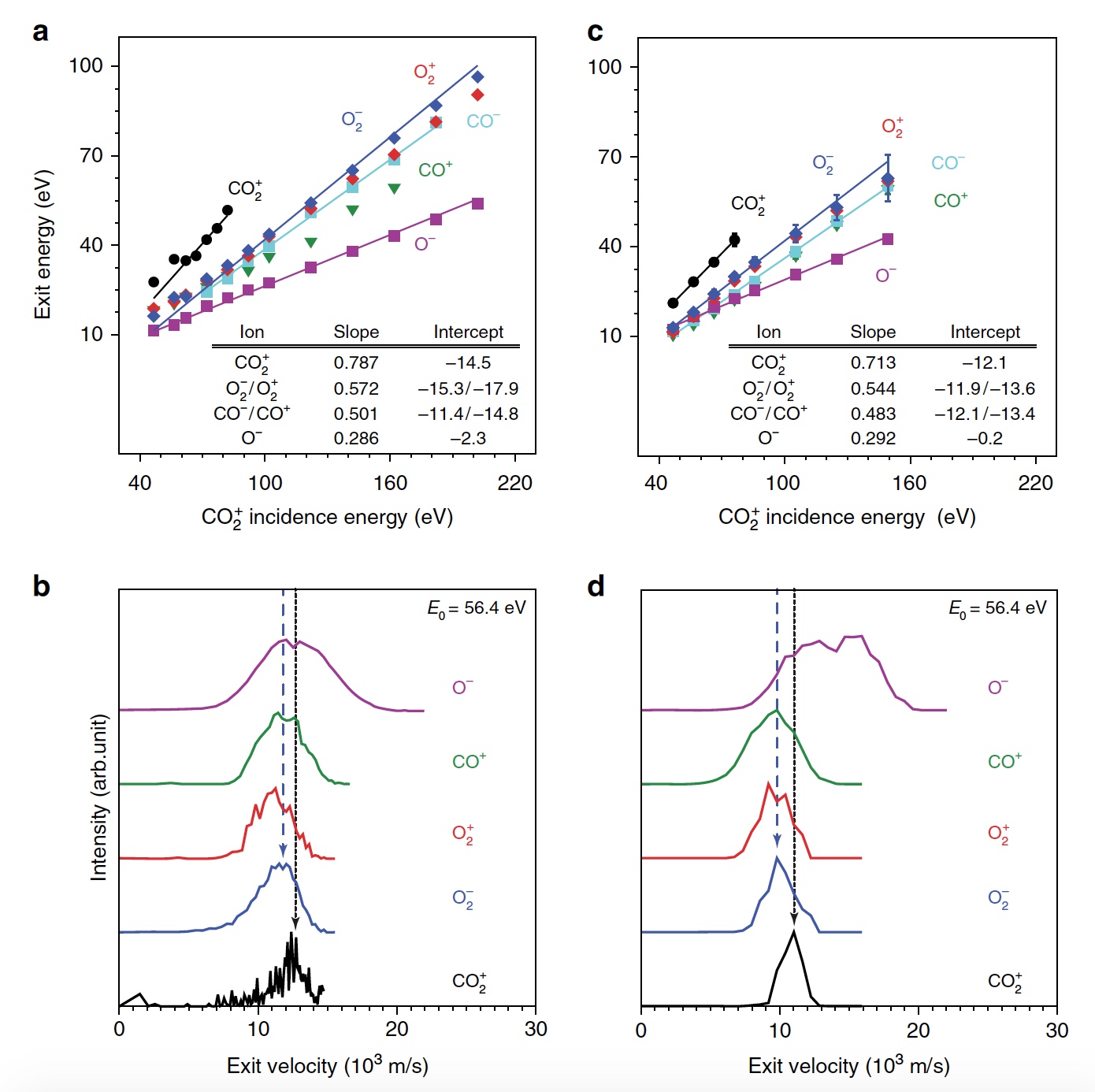
Image number 2
Next, scientists applied a model in which an O atom first collides with a surface Au atom, and then a second CO fragment collides without a rapid dissociation of the CO 2 molecule. The application of the BTS to this model of successive collisions gives a kinematic coefficient of 0.7870, which is in excellent agreement with the data on the energy of the outgoing CO 2 + (black line at 2a ). Graph 2a also shows the energies of the other exiting scattered ionic products.
The main potential source of such products can be called partial or complete dissociation of CO 2 and surface spraying of adsorbed CO 2 fragments. Despite the fact that some sputtering is indeed observed at high values of E 0 (> 140 eV), the kinematic analysis of the data on the energy of the outgoing particles provides convincing evidence of the presence of the pulsed dissociation of the CO 2 molecule.
Next, scientists conducted an analysis of the speed of the observed scattered particles.
Image 2b shows a comparison of the ion distribution peaks at E 0 = 56.4 eV. As we can see, the exit rates of scattered CO + , O 2 + , O 2 - and the slower part of the O - distribution overlap, indicating the common origin. However, the distribution of O is much wider, extending to higher output speeds, which may indicate an alternative origin. Ionic O 2 products come out at speeds lower than CO 2 + due to the inelasticity caused by the breaking of chemical bonds and non-resonant surface ionization.
The above-described kinematic analysis, as scientists say, gave exhaustive evidence that some CO 2 molecules are scattered intact after a two-step successive collision of O and CO fragments. However, questions still remain regarding various aspects of the O 2 education mechanism.
After the experiments, a number of questions remain. First, it remains unclear whether O 2 is formed using an electronic adiabatic or non-adiabatic mechanism. Secondly, the collision paths that underlie the velocity distribution at the exit of the ion fragments are unknown. Thirdly, it is unknown how much neutral O 2 is formed .
In this case, modeling, namely the method of classical molecular dynamics (MD method) can clarify the situation.
In the simulation of the CO 2 scattering path on Au (111), the scattering geometry observed in the experiment was applied. In this case, it is assumed that CO 2 is released on the surface of the potential energy of the singlet electron in the case of neutralizing the incoming CO 2 + ions before a hard collision.
Rapid neutralization occurs through resonant tunneling of electrons from the metal surface to the molecular cation, since the molecular level of CO 2 (-13.8 eV) is within the occupied Au zone (from -5.3 to -15.3 eV). The simulation also took into account electron transfer from / to the surface to take into account the ionization of neutral products of collisions.
Figure 2c shows the calculated energy values of the outgoing particles.
It was found that a small amount of CO 2 maintains integrity at E 0 > 80 eV, which is consistent with the absence of a signal at these energies during practical experiments. The data obtained through simulation are in excellent agreement with the experimental data, which is easy to see by comparing the graphs 2a (experiment) and 2c (simulation).
In addition to this, agreement between experiments and modeling is also manifested in comparing the speed of the output ions at E 0 = 56.4 eV ( 2d ). Both the model and the experiment show an expansion of the velocity distribution of CO + and O - . It was also confirmed that the O 2 + and O 2 - distributions are similar to the cation leaving more slowly than the anion. In addition, in both cases, it was found that CO 2 + is released at a faster rate than ionized O 2 products.
Consequently, the use of this simulation technique can provide fairly accurate data regarding the reaction mechanism of direct conversion of CO 2 to O 2 .
During the main stage of the simulation, 20,000 CO 2 –Au – Au collisions were reconstructed at different values of the energy of the outgoing particles. As a result, several variants of dissociation products were obtained, including O 2 ( 3a ).
Image number 3
Image 3b shows a representative trajectory resulting in the formation of O 2 . As a result of the pulsed energy transfer during the collision inside the rebounding CO 2 , a significant intramolecular rearrangement occurs. The O – O distance decreases, and the C – O distance increases, reaching a peak when CO 2 acquires a triangular configuration with almost equal bond lengths. Such a strongly bent intermediate CO 2 product has a significant amount of internal energy and quickly dissociates, resulting in the formation of a free C atom and a vibrational hot O 2 molecule.
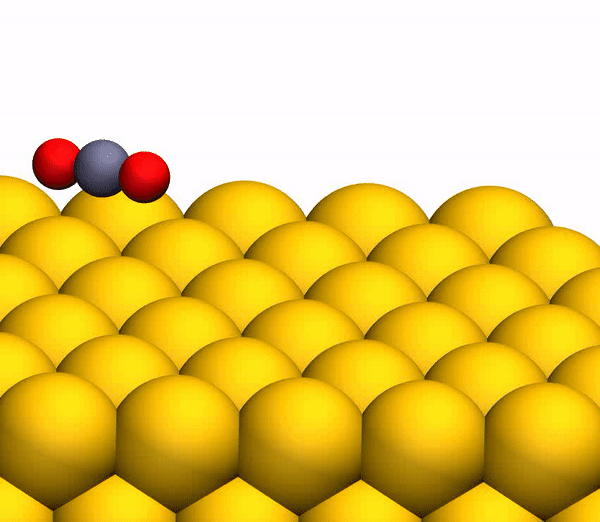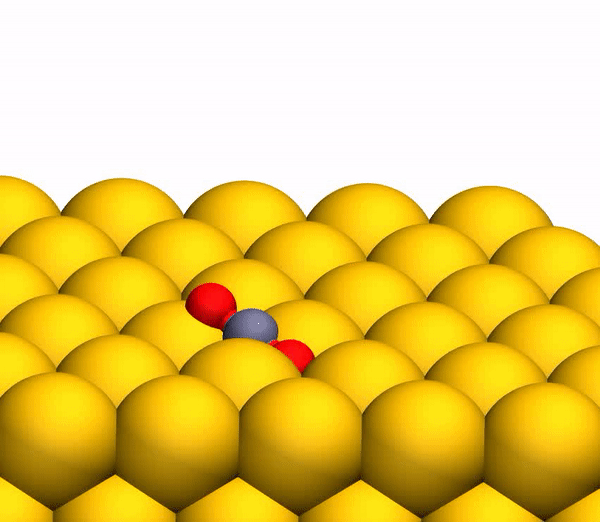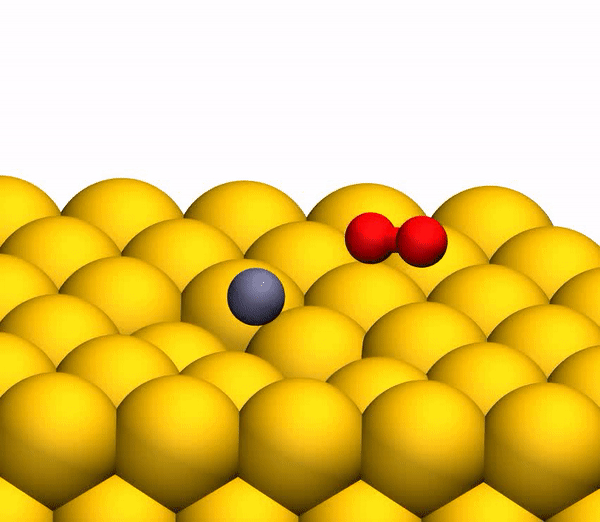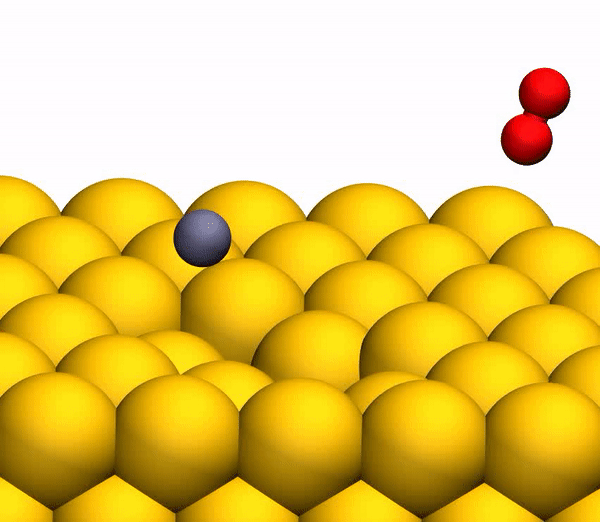
The process of separating CO 2 into a free C atom and a vibrationally hot O 2 molecule.
It is worth noting that the separation mechanism described above is fully consistent with the conclusions of the kinematic model used earlier.
Calculations have shown that approximately 5% of all trajectories lead to a strongly curved intermediate state of CO 2 , which is one of the stages of O 2 release. This state is fragmented mainly by partial dissociation (51%), followed by complete dissociation again, but with a higher result (33%).
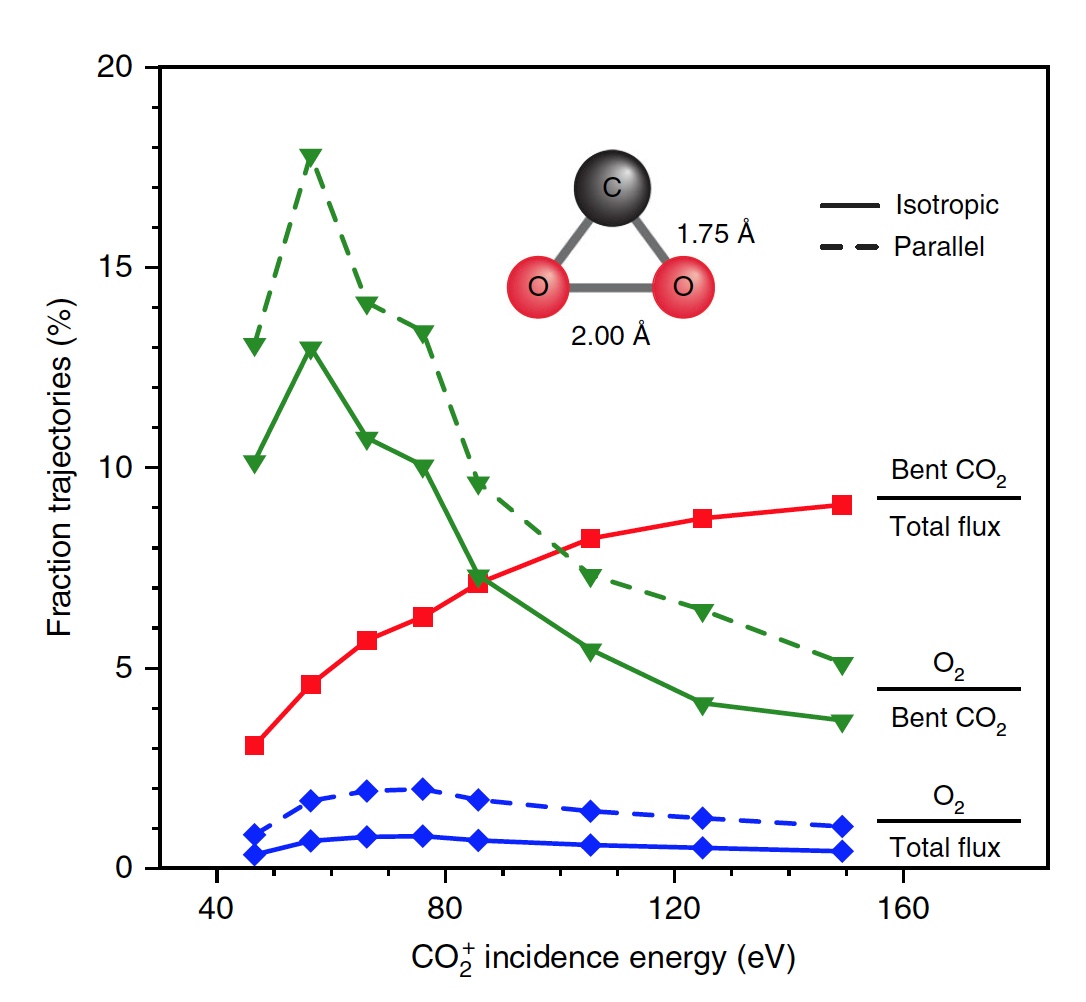
Image number 4
Scientists note that only 13 percent of all bent molecules of CO 2 produce oxygen. That is, the total result of the dissociation of CO 2 → C + O 2 is 0.6% at E 0 = 56.4 eV. If you increase the energy of the incident particles, you can get O 2 to 0.8 ± 0.2% with E 0 ~ 70 ± 15 eV (the blue line in image No. 4).
In addition, the share of O 2 forming trajectories increases significantly when a strongly curved intermediate state of CO 2 is reached (green line), reaching a maximum of 13% at E 0 ~ 55 ± 10 eV. The smaller total yield of neutral O 2 is due to the fact that only a small fraction of linear CO 2 molecules reaches a strongly bent state (red line). If we specifically change the orientation of the incoming CO 2 molecules (axis parallel to the surface), then as a result, 2% more O 2 can be obtained (dashed green line).
For a more detailed acquaintance with the nuances of the study I recommend to look into the report of scientists .
Epilogue
This work has demonstrated that non-standard chemical reactions can produce oxygen from carbon dioxide. No one will argue that this technique requires further refinements and improvements. However, one cannot deny the fact that this work really opens up new opportunities for interplanetary travel, the colonization of Mars and not only. There is also a problem with oxygen and carbon dioxide on our home planet. Rather, with the latter, for its volume has increased significantly since the moment of industrialization. And considering that CO 2 is one of the main culprits of global warming, reducing its amount and getting oxygen from it sounds like a very tempting idea. Since we cannot do without factories, factories, machines with internal combustion engines and others, so far, new methods of cleaning our atmosphere are not just necessary, but extremely necessary. Because traveling to distant planets is certainly cool, but before the mass transfer to Mars is still very, very far away, therefore, it is worth thinking more about the Earth on which we live, than about Mars, about which we dream.
Thank you for your attention, stay curious and have a good working week, guys! :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to friends, 30% discount for Habr's users on a unique analogue of the entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to share the server? (Options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to build an infrastructure building. class c using servers Dell R730xd E5-2650 v4 worth 9000 euros for a penny?
Source: https://habr.com/ru/post/454810/
All Articles