HBO, thank you for reminding me ... "Chernobyl first-aid kit" of a Belarusian pharmacist
Whatever they say - we will not argue
Whatever gave - we will not believe
Egor Letov "Like a flyer"
I think it is not worth talking about the sensational Chernobyl series and the effectiveness of such a “serial” impact on the masses. Especially the masses living in the territories shown in the film. The release of each new series is accompanied by a surge of publications in the FB. In each of which bitterness, fear, pain. What can I do in such a situation ("who is to blame and what to do?")? I can only describe my view on radiation therapy. Thank you dear Department of High Energy Chemistry and prof. Shadyro O.I. who nurtured us in their laboratories, waste medicine radiochemists. I hope with my article the honor of this once legendary department I will not defame.
Well, I write, I write, because they began to forget ... Fearfully, they quickly began to forget. First, potassium iodide disappeared in pharmacies (I’m not even talking about the antidotes described in the article), then benefits from liquidators, knowledge from people, etc. also inevitably disappeared. etc.
')
In general, thank you, the HBO screenwriters, for stirring Memory. My feasible contribution - under the cut. Rating available (and not very) antidotes that can trigger with radiation release. Bookmark - put strictly ALL! And I read it myself - send it to a friend.

At the beginning of the article I will voice my opinion on nuclear power in general. It is steadfastly positive. I believe that nuclear reactors can and should be actively used, because until humanity has invented the best source of energy. But. But I am an ardent opponent of disorder, concealment, silence and reckless ideologized stupidity when working with nuclear power plants. How much can you still close the “embarrassment of tyranny” with the bodies of innocent young men ... It is unacceptable to build and operate facilities like nuclear power plants using “Stakhanov’s methods” using forced labor and putting people who have pulled out for a long ruble to be operators. Because, as always, it will end sadly. And those who are least to blame will suffer.
Okay, go to the topic. Despite the fact that the article in each poison - its own antidote. How to escape or at least try (upd: about antidotes for household poisoning) I generally considered the most common antidotes, now I decided to focus on working with radionuclides. In order to push off from something, let's take the main contaminants of the Chernobyl NPP:
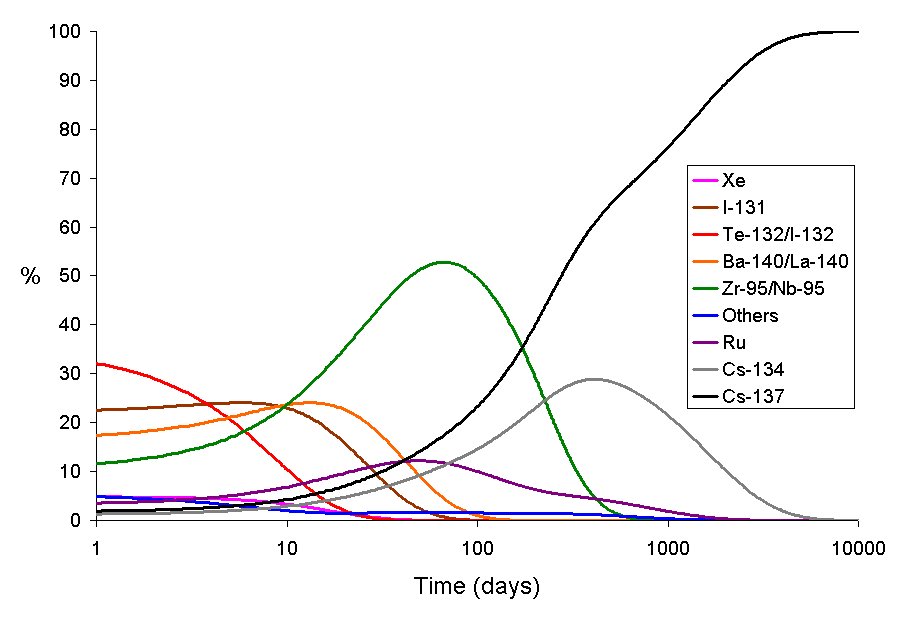
We do not take into account extreme options, such as extinguishing a burning reactor or piloting a helicopter over it, since, after all, this is more likely an exception. Most of the victims are in areas that are contaminated by radionuclides through a) movement of air masses with radioactive dust and closely related b) radioactive fallout and finally c) food intakes grown in areas affected by the first two points.
In cities, the bulk of hazardous substances will accumulate on flat surface areas: lawns, roads, roofs. In the case of agricultural land, radioactive substances will be deposited on the leaves of plants and on the grass (and then migrate to herbivores). Gradually, radionuclides from the leaves, along with rain or fallen leaves, enter the soil, accumulate there and slowly but surely, through the root system, enter agricultural plants. It is clear that the main accumulator of radionuclides is forests. Due to the constant circulation of certain radionuclides (without becoming insoluble), the pollution levels of forest products (mushrooms, berries and game) remain quite high and long after the release. The same pattern holds true for drainless lakes (and for fish in them).
Accordingly, domestic measures to combat radiation can be divided into two main areas:
The radioactive isotopes of plutonium and americium can persist in the soil for hundreds, perhaps thousands of years, but their number is small and can be neglected.
As I said, the main short-lived isotope that strikes the thyroid gland is iodine-131. Iodine, as is well known, has the property of excellent sublimation (it passes from the solid form directly into a gas) and, in a volatile aerosol form, spread by the wind. The main threat from this isotope exists during the week after the accident (and therefore prompt information is vital !). The best antidote is stable iodine, existing in the form of potassium iodide tablets. It is not for nothing that this known substance is hidden in the well-known AI-2 field medicine cabinet under the name "radioprotective agent No. 2" Iodine accumulates very unevenly in the body, about half of it is deposited in the thyroid gland. Therefore, for prophylactic purposes, it is necessary to saturate the body with stable, safe iodine and not give a single chance to iodine radioactive. The most practical option is potassium iodide tablets. But for example, in Minsk today this drug is not realistic to find, a couple of pharmacies have it on sale for 2 million cities. The analogue is the drug iodomarin , which can be used to prevent accumulation in the thyroid gland, both in the case of inhalation of radioactive iodine, and when it enters the digestive tract. Traditional prophylactic dosage is 125 mg for adults and children over 2 years old (40 mg for children under 2 years of age). In the absence of the tablet form, it is allowed to use a 5% alcoholic solution of iodine , or Lugol solution . Adults and adolescents over the age of 14 take 40 drops of a 5% solution of iodine (20 drops 2 times) or 20 drops of Lugol's solution (10 drops 2 times) per day. For children over 5 years old, 20 drops of 5% solution of iodine (10 drops 2 times) or 10 drops of Lugol solution (5 drops, 2 times) per day. Children under 5 years old do not use the alcoholic solution of iodine and the Lugol solution inside. For them, iodine prophylaxis can be carried out by applying a swab to the skin of the forearm or lower leg of an alcoholic solution of iodine (in view of possible burns better than 2.5% solution) in the form of strips or a mesh in the following doses: children 2 to 5 years old 20 drops, children under 2 years old - 10 drops per skin once a day.
The recommended dose of 125 mg of potassium iodide is more than 1000 times the daily need of the body for a trace element such as iodine. The protective effect is observed due to the pharmacological blockade of the synthesis of thyroxin in the thyroid gland. The mentioned dose of iodine ions up to 99.5% eliminates the penetration of radioactive iodine-131 into the thyroid gland (the reaction is observed already after 5 minutes after taking a potassium iodide tablet on an empty stomach and 30 minutes on a full stomach). The effectiveness of protection begins to decline 1.5–2 days after the first dose. The time for complete recovery from the blockade of thyroxin synthesis in the thyroid gland is noted when taking potassium iodide at a dose of 2–200 mg in 7–24 days. The effectiveness of potassium iodide quickly decreases when it is taken several hours after the intake of radioactive iodine into the body: after 1 hour - by 1/4, after 2 hours - by 1/3, from 3 to 5 hours - by 50% and after 8 hours it missing. For this reason, the delay in carrying out iodine prophylaxis for more than 6 hours after the fallout of radioactive fallout sharply limits its effectiveness, and a day later it is doubtful in its expediency. The effectiveness of iodine prophylaxis is quite high (90-100%) when taking iodine preparations before or within 30 minutes from the start of inhalation of aerosols of radioactive iodine. 2 hours after radioactive iodine is introduced into the body, the effectiveness of iodine prophylaxis sharply decreases (by 1/3); in a day it does not affect the cumulation of the radiation dose from the previous incorporation of radioactive iodine. That is why LIVING IMPORTANT IS THE RATE OF INFORMATION OF THE POPULATION. In the literal sense of the word, every minute of delay = someone's agonizing illness / death ...
Iodine preparations are used until the threat of radioactive isotopes of iodine enters the body. However, it is worth noting that in the mass consciousness, the population has already developed a conditioned reflex - with any mention of radiation, they immediately buy iodine in pharmacies. This does not make much sense, it is enough to have the required amount for all family members for a week. Well, considering what was said in the paragraph above, you can't do it for the future if you are late with the reception. Iodine, whatever one may say, is still a halogen and can cause serious poisoning during an overdose. The best option is potassium iodide tablets / powder. Iodine tincture, in addition to the useful iodide contains molecular iodine, which, before being absorbed, must be converted into ions. Therefore, a good option if there is no pure KI is to “extinguish” the measured amount of iodine tincture with sodium thiosulfate solution before bleaching, and then use it (advice from jar_ohty ).
How to do / what to replace?
At one time, in the article Notes phytochemist. Banana peel strikes back. I described such an interesting class of nutrients as anti-nutrients . In their subset there are the following:
So, in the most extreme case, if you can’t find iodine preparations at all, you can either a) eat foods that are high in iodine (seaweed, cod and its liver, shrimp, tuna, prunes, feijoa, persimmon ) b) have goytrog containing products that block the intake of iodine in the thyroid in general (soy, broccoli).
The most unpleasant long-lived isotopes can be considered cesium-137 and strontium-90. Cesium-137 is present in emissions from an accident at any nuclear facility, from Chernobyl and Fukushima to some hypothetical " dirty bomb " anywhere.
The antidote to cesium-137 from water is a mixture of potassium hexacyanoferrates (II) - Prussian blue (“ferrocin”). It binds cesium, which comes with food and / or water inside the gastrointestinal tract and does not allow it to be absorbed into the blood and enter the body. As a result, almost 100% of cesium-137 passes through the gastrointestinal tract without stopping. Further I will quote my own article in which this antidote already mentioned:
Application: the recommended dose of 1 g of ferrocyanide 3 times a day in case of a threat or actual intake of cesium, rubidium and other fission products of transuranium elements into the body, as well as in the case of inability to exclude the entry of radioactive cesium into the gastrointestinal tract with water or food. " The drug reduces the half-life of radioactive cesium in humans by 2-4 times when it is taken orally 1 g 3 times a day, every day for 2-4 weeks.
Of foreign analogues of ferocin-containing preparations, there is Radiogardase (Germany). In general, ferrocyanides are absent more than completely in Belarusian pharmacies. At the end of the article "school laboratory work" on the preparation of Prussian blue at home.
Potassium acetate , which can be obtained by neutralizing potassium alkali (or some potash from ash) with acetic acid, can also act as a contact antidote detoxicant for cesium-137. I certainly understand that amateur work, but who knows how life will turn. It is recommended to take 1 tablespoon of a 15% solution 5 times a day.
Of the drugs for humans, polysurmine is also worth mentioning - to remove strontium isotopes - first and foremost, strontium-90, which has a close half-life (slightly less than 30 years) with cesium -137 and a fraction of the yield from the division of nuclear fuel, i.e. its amount in the reactor. These two factors explain why, in addition to pollution maps from the Chernobyl accident, there are similar maps for strontium pollution. The mentioned antidote is also unrealistic to find in pharmacies. In fact, it is an antimony-silicon cation exchanger, which is obtained by the reaction of antimony chloride (V) with calcium silicate . The main difficulty is the search for antimony chloride , since the component is quite active and rare. Calcium silicate is also used in the construction of fireplaces. (Promasil, Silca) or as a filler in the production of building materials. In addition, the natural mineral wollastonite is practically pure calcium silicate. There is, by the way, an easier way to obtain this sorbent (RF patent No. 2324535 ):
If you managed to synthesize / get / buy - use the following method. Inside in a dose of 4 g in 0.5 glass of water at the time of eating 3 times a day for 7 days
In addition to polysurmine, adsobar can also be mentioned - barium sulfate, which is recommended to be taken in the amount of 30 g / cup of water. This enterosorbent is indicated in the event of a threat or actual intake of strontium radioisotope inside the body.
How to do / what to replace?
Barium sulfate is actively used as a glue paint, since it is insoluble in organic solvents. White on its basis is cheaper than lead, non-toxic and does not darken from hydrogen sulfide. Barium sulfate is the standard of whiteness. The crystalline barium sulphate obtained by precipitation with sodium sulphate from a solution of barium sulphide is also called a blanc fiks (fr. Blanc fixe). Very widely this enterosorbent is used as a radiopaque substance, in X-ray studies of the gastrointestinal tract. For an x-ray examination of the digestive organs, the patient ingests a suspension of barium sulfate (“barite porridge”) with a barium content of 58.7%. It was this substance that was added to Lego products in the 90s to ensure radiopacity in case a child swallows a part. True, the experiment ended in failure due to the reduction in the strength of parts.
The mode of use of barium sulfate: the drug is effective when used prophylactically for 1-2 hours before the intake of the radionuclide and as an emergency in the coming hours after the intake of the radionuclide. Apply orally once in a dose of 25 g of powder, pre-mixed it in a glass of water. In the case of the use of the drug as a means of simple assistance in case of radionuclide poisoning, it is prescribed together with laxatives (magnesium sulphate ~ magnesia or sodium sulphate ~ glauber's salt - 25 g).
In addition to the aforementioned enterosorbent, sodium alginate ( Algisorb enterosorbent = calcium alginate) can also be used to eliminate both cesium and strontium from the body. This reagent is registered in the food industry as a food additive E401, used as a thickener and stabilizer. You can easily buy it even in Minsk (it is used for making handmade pastila , which, indeed, will turn out to be incredibly useful and radioprotective).
Application: in humans, the protective effect of alginate in a dose of 20 g in relation to radioactive strontium is 90%. Reception on 0,5 g at food intake in a dose of 5 g 3 times a day. In case of acute poisoning with radioactive strontium, the dose of the drug increases to 20 g.
If sodium alginate could not be found, look for pectin (often sold with spices). As an enterosorbent (suspension in water), 5 g is applied 3 times a day. The most extreme option is candied fruit (orange zest is boiled in sugar syrup, followed by the addition of citric acid to taste). Dose - similar to pectin (more-better, natural material after all).
Note: remembered as my supervisor, the late academician V.S. Komarov , talking about the orientation of the institute and the laboratory (adsorbents) to combat radiation in food, often recalled the case of contaminated meat. Saying a sack with natural zeolite from Georgian deposits was thrown into a meat pot, they cooked and as a result, the meat gave a much smaller background than the natural one. It really is. Natural zeolites and bentonites are capable of selectively removing radionuclides while maintaining ion exchange properties under conditions of high radioactivity. Selective sorption of natural zeolites is arranged in the following descending order: 90Sr> 137Cs> 60Co> 45Ca. So, it makes sense to buy cats a bentonite aggregate instead of sawdust. In which case - double assignment will work.
Note: it is not necessary to take bentonite orally (this is due to the ability of these clays to swell in a humid environment), it is best to use it for cooking along with the products. Inside you can zeolites (molecular sieves), which also have enterosorption properties and for this purpose, by the way, are actively used in animal feed.
Various complexing agents (DTPA, EDTA, etc.) are used as substances that accelerate the excretion of radionuclides without clearly selected specificity. Those. At least the packaging of Trilon-B should always be at home. The component is inexpensive and widely available, both in domestic stores and in foreign auctions. We can mention the missing pentacin everywhere, it’s also calcium trisodium pentetate, aka Ca-DTPA (pentetate calcium trisodium). This reagent is an excellent complexing agent, accelerates the excretion of plutonium, yttrium, cerium, zinc, cadmium, cobalt, manganese and lead, including their radioactive isotopes. True, you can only buy abroad (not surprising, because there and "Chernobyl" removed ...). Of the drugs available (so far) for us, we can recall unitiol , which is used in case of poisoning with mercury, arsenic, chromium and other “thiol poisons”. Unithiol forms complexes including. with radioactive polonium ( "the Litvinenko case" ). The drug (5% solution) is administered intramuscularly in the amount of 5-10 ml (at the rate of 1 ml of 5% solution per 10 kg of patient weight) on the first day every 6-8 hours (3-4 injections per day) , on the second day, 2–3 injections in 9–12 hours; in the following days, 1–2 injections per day.
Indirectly (due to functional groups with sulfur), such a legendary drug as cystamine (I read about it only in old books on civil defense) is associated with unithiol, referring to ... see below. As a replacement, I can offer acetylcysteine , which is used as a cough aid . And there is still a pharmacy in the city :)
The “heavy artillery” includes radioprotectors , i.e. chemical protection against radiation. The most high-speed radioprotectors are drugs that have vasoconstrictive properties. First of all, we can mention the so-called. " preparation B-190 " is a means of emergency medical protection with external radiation exposure. Substitute indralin , an imidazole derivative, an agonist of a- adreno - reactive structures of the body, is intended for use in extreme situations accompanied by the threat of exposure in doses of more than 1 Gy to reduce the severity of acute radiation damage to the body. The drug is administered orally at a dose of 0.45 g (3 tablets of 0.15 g) for 10-15 minutes before the intended exposure. The duration of the radioprotector is about 1 hour. It is almost impossible to find. Another effective radioprotector from the imidazoline group is naphthyzine . Introduced in a volume of 1 ml for 3-5 minutes before the intended exposure. It is true that in our pharmacies, banal drops in the nose are more common ... In addition, there is one nuance. The use of radioprotectors for short-term irradiation at doses of less than 1 Gy is impractical because of the absence of a practically significant anti-radiation effect under these conditions.
To protective drugs can be attributed, and drugs that increase the body's resistance to radiation due to the ability for a sufficiently long period to increase the so-called "endogenous background of radiation resistance." «» ( ) ( , , ) «» ( ) «». , ( ) , . . - ( ). — ( ). ( , ). , , . 24 : 15 (75 ).
: ( — , , , — : - «», «», «», «» ..), – . 14-21 2-3 20-30 30 3 . : () . . , , .
That's all. (, « » ), , , . — « » ;)
UPD : , Ark_V
NB / — FB . , .
Whatever gave - we will not believe
Egor Letov "Like a flyer"
I think it is not worth talking about the sensational Chernobyl series and the effectiveness of such a “serial” impact on the masses. Especially the masses living in the territories shown in the film. The release of each new series is accompanied by a surge of publications in the FB. In each of which bitterness, fear, pain. What can I do in such a situation ("
Well, I write, I write, because they began to forget ... Fearfully, they quickly began to forget. First, potassium iodide disappeared in pharmacies (I’m not even talking about the antidotes described in the article), then benefits from liquidators, knowledge from people, etc. also inevitably disappeared. etc.
')
In general, thank you, the HBO screenwriters, for stirring Memory. My feasible contribution - under the cut. Rating available (and not very) antidotes that can trigger with radiation release. Bookmark - put strictly ALL! And I read it myself - send it to a friend.

At the beginning of the article I will voice my opinion on nuclear power in general. It is steadfastly positive. I believe that nuclear reactors can and should be actively used, because until humanity has invented the best source of energy. But. But I am an ardent opponent of disorder, concealment, silence and reckless ideologized stupidity when working with nuclear power plants. How much can you still close the “embarrassment of tyranny” with the bodies of innocent young men ... It is unacceptable to build and operate facilities like nuclear power plants using “Stakhanov’s methods” using forced labor and putting people who have pulled out for a long ruble to be operators. Because, as always, it will end sadly. And those who are least to blame will suffer.
Okay, go to the topic. Despite the fact that the article in each poison - its own antidote. How to escape or at least try (upd: about antidotes for household poisoning) I generally considered the most common antidotes, now I decided to focus on working with radionuclides. In order to push off from something, let's take the main contaminants of the Chernobyl NPP:

We do not take into account extreme options, such as extinguishing a burning reactor or piloting a helicopter over it, since, after all, this is more likely an exception. Most of the victims are in areas that are contaminated by radionuclides through a) movement of air masses with radioactive dust and closely related b) radioactive fallout and finally c) food intakes grown in areas affected by the first two points.
In cities, the bulk of hazardous substances will accumulate on flat surface areas: lawns, roads, roofs. In the case of agricultural land, radioactive substances will be deposited on the leaves of plants and on the grass (and then migrate to herbivores). Gradually, radionuclides from the leaves, along with rain or fallen leaves, enter the soil, accumulate there and slowly but surely, through the root system, enter agricultural plants. It is clear that the main accumulator of radionuclides is forests. Due to the constant circulation of certain radionuclides (without becoming insoluble), the pollution levels of forest products (mushrooms, berries and game) remain quite high and long after the release. The same pattern holds true for drainless lakes (and for fish in them).
Accordingly, domestic measures to combat radiation can be divided into two main areas:
- Prevention of infection with short-lived isotopes, where the greatest danger is represented by radioactive iodine (half-life period of eight days), and tellurium
- Fighting radionuclides with a half-life of about 30 years accumulating in water / soil / food.
The radioactive isotopes of plutonium and americium can persist in the soil for hundreds, perhaps thousands of years, but their number is small and can be neglected.
The fight against short-lived isotopes
As I said, the main short-lived isotope that strikes the thyroid gland is iodine-131. Iodine, as is well known, has the property of excellent sublimation (it passes from the solid form directly into a gas) and, in a volatile aerosol form, spread by the wind. The main threat from this isotope exists during the week after the accident (and therefore prompt information is vital !). The best antidote is stable iodine, existing in the form of potassium iodide tablets. It is not for nothing that this known substance is hidden in the well-known AI-2 field medicine cabinet under the name "radioprotective agent No. 2" Iodine accumulates very unevenly in the body, about half of it is deposited in the thyroid gland. Therefore, for prophylactic purposes, it is necessary to saturate the body with stable, safe iodine and not give a single chance to iodine radioactive. The most practical option is potassium iodide tablets. But for example, in Minsk today this drug is not realistic to find, a couple of pharmacies have it on sale for 2 million cities. The analogue is the drug iodomarin , which can be used to prevent accumulation in the thyroid gland, both in the case of inhalation of radioactive iodine, and when it enters the digestive tract. Traditional prophylactic dosage is 125 mg for adults and children over 2 years old (40 mg for children under 2 years of age). In the absence of the tablet form, it is allowed to use a 5% alcoholic solution of iodine , or Lugol solution . Adults and adolescents over the age of 14 take 40 drops of a 5% solution of iodine (20 drops 2 times) or 20 drops of Lugol's solution (10 drops 2 times) per day. For children over 5 years old, 20 drops of 5% solution of iodine (10 drops 2 times) or 10 drops of Lugol solution (5 drops, 2 times) per day. Children under 5 years old do not use the alcoholic solution of iodine and the Lugol solution inside. For them, iodine prophylaxis can be carried out by applying a swab to the skin of the forearm or lower leg of an alcoholic solution of iodine (in view of possible burns better than 2.5% solution) in the form of strips or a mesh in the following doses: children 2 to 5 years old 20 drops, children under 2 years old - 10 drops per skin once a day.
The recommended dose of 125 mg of potassium iodide is more than 1000 times the daily need of the body for a trace element such as iodine. The protective effect is observed due to the pharmacological blockade of the synthesis of thyroxin in the thyroid gland. The mentioned dose of iodine ions up to 99.5% eliminates the penetration of radioactive iodine-131 into the thyroid gland (the reaction is observed already after 5 minutes after taking a potassium iodide tablet on an empty stomach and 30 minutes on a full stomach). The effectiveness of protection begins to decline 1.5–2 days after the first dose. The time for complete recovery from the blockade of thyroxin synthesis in the thyroid gland is noted when taking potassium iodide at a dose of 2–200 mg in 7–24 days. The effectiveness of potassium iodide quickly decreases when it is taken several hours after the intake of radioactive iodine into the body: after 1 hour - by 1/4, after 2 hours - by 1/3, from 3 to 5 hours - by 50% and after 8 hours it missing. For this reason, the delay in carrying out iodine prophylaxis for more than 6 hours after the fallout of radioactive fallout sharply limits its effectiveness, and a day later it is doubtful in its expediency. The effectiveness of iodine prophylaxis is quite high (90-100%) when taking iodine preparations before or within 30 minutes from the start of inhalation of aerosols of radioactive iodine. 2 hours after radioactive iodine is introduced into the body, the effectiveness of iodine prophylaxis sharply decreases (by 1/3); in a day it does not affect the cumulation of the radiation dose from the previous incorporation of radioactive iodine. That is why LIVING IMPORTANT IS THE RATE OF INFORMATION OF THE POPULATION. In the literal sense of the word, every minute of delay = someone's agonizing illness / death ...
Iodine preparations are used until the threat of radioactive isotopes of iodine enters the body. However, it is worth noting that in the mass consciousness, the population has already developed a conditioned reflex - with any mention of radiation, they immediately buy iodine in pharmacies. This does not make much sense, it is enough to have the required amount for all family members for a week. Well, considering what was said in the paragraph above, you can't do it for the future if you are late with the reception. Iodine, whatever one may say, is still a halogen and can cause serious poisoning during an overdose. The best option is potassium iodide tablets / powder. Iodine tincture, in addition to the useful iodide contains molecular iodine, which, before being absorbed, must be converted into ions. Therefore, a good option if there is no pure KI is to “extinguish” the measured amount of iodine tincture with sodium thiosulfate solution before bleaching, and then use it (advice from jar_ohty ).
How to do / what to replace?
At one time, in the article Notes phytochemist. Banana peel strikes back. I described such an interesting class of nutrients as anti-nutrients . In their subset there are the following:
Glucosinolates (the same, sharp, horseradish, mustard, etc.) prevent iodine absorption, thereby suppressing the function of the thyroid gland, and therefore are considered goitrogens (or goitrogenic substances - substances that contribute to the formation of goiter).
So, in the most extreme case, if you can’t find iodine preparations at all, you can either a) eat foods that are high in iodine (seaweed, cod and its liver, shrimp, tuna, prunes, feijoa, persimmon ) b) have goytrog containing products that block the intake of iodine in the thyroid in general (soy, broccoli).
Fighting long-lived isotopes
The most unpleasant long-lived isotopes can be considered cesium-137 and strontium-90. Cesium-137 is present in emissions from an accident at any nuclear facility, from Chernobyl and Fukushima to some hypothetical " dirty bomb " anywhere.
The antidote to cesium-137 from water is a mixture of potassium hexacyanoferrates (II) - Prussian blue (“ferrocin”). It binds cesium, which comes with food and / or water inside the gastrointestinal tract and does not allow it to be absorbed into the blood and enter the body. As a result, almost 100% of cesium-137 passes through the gastrointestinal tract without stopping. Further I will quote my own article in which this antidote already mentioned:
For example, the antidote for thallium is Prussian blue , or Prussian blue. Chemist to get the blue of azure is not difficult, the benefit of " yellow blood salt " and ferric salts are almost everywhere. Ferrotsin tablets can help the man in the street (initially positioned as a sorbent of radioactive cesium), which after the Chernobyl disaster could still be found, and then suddenly it became difficult. In the extreme case, you can try to look for a veterinary drug Bifezh fed to animals in areas contaminated with radionuclides. As an emergency option - there is watercolor paint , where the azure blue is used as a blue pigment. Note that the yellow blood salt is a food additive E536, which is put, for example, in sprat in a Belarusian-made tomato , i.e. This piece can be quite legally purchased in stores selling food additives :)
Application: the recommended dose of 1 g of ferrocyanide 3 times a day in case of a threat or actual intake of cesium, rubidium and other fission products of transuranium elements into the body, as well as in the case of inability to exclude the entry of radioactive cesium into the gastrointestinal tract with water or food. " The drug reduces the half-life of radioactive cesium in humans by 2-4 times when it is taken orally 1 g 3 times a day, every day for 2-4 weeks.
Of foreign analogues of ferocin-containing preparations, there is Radiogardase (Germany). In general, ferrocyanides are absent more than completely in Belarusian pharmacies. At the end of the article "school laboratory work" on the preparation of Prussian blue at home.
Potassium acetate , which can be obtained by neutralizing potassium alkali (or some potash from ash) with acetic acid, can also act as a contact antidote detoxicant for cesium-137. I certainly understand that amateur work, but who knows how life will turn. It is recommended to take 1 tablespoon of a 15% solution 5 times a day.
Of the drugs for humans, polysurmine is also worth mentioning - to remove strontium isotopes - first and foremost, strontium-90, which has a close half-life (slightly less than 30 years) with cesium -137 and a fraction of the yield from the division of nuclear fuel, i.e. its amount in the reactor. These two factors explain why, in addition to pollution maps from the Chernobyl accident, there are similar maps for strontium pollution. The mentioned antidote is also unrealistic to find in pharmacies. In fact, it is an antimony-silicon cation exchanger, which is obtained by the reaction of antimony chloride (V) with calcium silicate . The main difficulty is the search for antimony chloride , since the component is quite active and rare. Calcium silicate is also used in the construction of fireplaces. (Promasil, Silca) or as a filler in the production of building materials. In addition, the natural mineral wollastonite is practically pure calcium silicate. There is, by the way, an easier way to obtain this sorbent (RF patent No. 2324535 ):
... a method of obtaining antimony-silicon sorbent, which includes treating trivalent antimony oxide in solid form with a solution of hydrochloric acid while simultaneously introducing hydrogen peroxide solution, mixing with alkali metal metasilicate in the presence of monosodium phosphate to form a gel, maturing the gel, washing it with a solution of sodium chloride, baking soda, mixing for 1.5-3.0 hours, drying to form a xerogel, grinding, treatment with nitric acid, holding for 1.5-3.0 hours, washing in ode, drying and grinding to a particle size of the sorbent 10-4-10 -5 microns.Antimony, by the way, can be easily purchased on aliexpress , for quite affordable ~ $ 50. All other reagents are widely available.
If you managed to synthesize / get / buy - use the following method. Inside in a dose of 4 g in 0.5 glass of water at the time of eating 3 times a day for 7 days
In addition to polysurmine, adsobar can also be mentioned - barium sulfate, which is recommended to be taken in the amount of 30 g / cup of water. This enterosorbent is indicated in the event of a threat or actual intake of strontium radioisotope inside the body.
How to do / what to replace?
Barium sulfate is actively used as a glue paint, since it is insoluble in organic solvents. White on its basis is cheaper than lead, non-toxic and does not darken from hydrogen sulfide. Barium sulfate is the standard of whiteness. The crystalline barium sulphate obtained by precipitation with sodium sulphate from a solution of barium sulphide is also called a blanc fiks (fr. Blanc fixe). Very widely this enterosorbent is used as a radiopaque substance, in X-ray studies of the gastrointestinal tract. For an x-ray examination of the digestive organs, the patient ingests a suspension of barium sulfate (“barite porridge”) with a barium content of 58.7%. It was this substance that was added to Lego products in the 90s to ensure radiopacity in case a child swallows a part. True, the experiment ended in failure due to the reduction in the strength of parts.
The mode of use of barium sulfate: the drug is effective when used prophylactically for 1-2 hours before the intake of the radionuclide and as an emergency in the coming hours after the intake of the radionuclide. Apply orally once in a dose of 25 g of powder, pre-mixed it in a glass of water. In the case of the use of the drug as a means of simple assistance in case of radionuclide poisoning, it is prescribed together with laxatives (magnesium sulphate ~ magnesia or sodium sulphate ~ glauber's salt - 25 g).
In addition to the aforementioned enterosorbent, sodium alginate ( Algisorb enterosorbent = calcium alginate) can also be used to eliminate both cesium and strontium from the body. This reagent is registered in the food industry as a food additive E401, used as a thickener and stabilizer. You can easily buy it even in Minsk (it is used for making handmade pastila , which, indeed, will turn out to be incredibly useful and radioprotective).
Application: in humans, the protective effect of alginate in a dose of 20 g in relation to radioactive strontium is 90%. Reception on 0,5 g at food intake in a dose of 5 g 3 times a day. In case of acute poisoning with radioactive strontium, the dose of the drug increases to 20 g.
If sodium alginate could not be found, look for pectin (often sold with spices). As an enterosorbent (suspension in water), 5 g is applied 3 times a day. The most extreme option is candied fruit (orange zest is boiled in sugar syrup, followed by the addition of citric acid to taste). Dose - similar to pectin (more-better, natural material after all).
Note: remembered as my supervisor, the late academician V.S. Komarov , talking about the orientation of the institute and the laboratory (adsorbents) to combat radiation in food, often recalled the case of contaminated meat. Saying a sack with natural zeolite from Georgian deposits was thrown into a meat pot, they cooked and as a result, the meat gave a much smaller background than the natural one. It really is. Natural zeolites and bentonites are capable of selectively removing radionuclides while maintaining ion exchange properties under conditions of high radioactivity. Selective sorption of natural zeolites is arranged in the following descending order: 90Sr> 137Cs> 60Co> 45Ca. So, it makes sense to buy cats a bentonite aggregate instead of sawdust. In which case - double assignment will work.
Note: it is not necessary to take bentonite orally (this is due to the ability of these clays to swell in a humid environment), it is best to use it for cooking along with the products. Inside you can zeolites (molecular sieves), which also have enterosorption properties and for this purpose, by the way, are actively used in animal feed.
Broad-spectrum complexing agents / radioprotectors
Various complexing agents (DTPA, EDTA, etc.) are used as substances that accelerate the excretion of radionuclides without clearly selected specificity. Those. At least the packaging of Trilon-B should always be at home. The component is inexpensive and widely available, both in domestic stores and in foreign auctions. We can mention the missing pentacin everywhere, it’s also calcium trisodium pentetate, aka Ca-DTPA (pentetate calcium trisodium). This reagent is an excellent complexing agent, accelerates the excretion of plutonium, yttrium, cerium, zinc, cadmium, cobalt, manganese and lead, including their radioactive isotopes. True, you can only buy abroad (not surprising, because there and "Chernobyl" removed ...). Of the drugs available (so far) for us, we can recall unitiol , which is used in case of poisoning with mercury, arsenic, chromium and other “thiol poisons”. Unithiol forms complexes including. with radioactive polonium ( "the Litvinenko case" ). The drug (5% solution) is administered intramuscularly in the amount of 5-10 ml (at the rate of 1 ml of 5% solution per 10 kg of patient weight) on the first day every 6-8 hours (3-4 injections per day) , on the second day, 2–3 injections in 9–12 hours; in the following days, 1–2 injections per day.
Indirectly (due to functional groups with sulfur), such a legendary drug as cystamine (I read about it only in old books on civil defense) is associated with unithiol, referring to ... see below. As a replacement, I can offer acetylcysteine , which is used as a cough aid . And there is still a pharmacy in the city :)
The “heavy artillery” includes radioprotectors , i.e. chemical protection against radiation. The most high-speed radioprotectors are drugs that have vasoconstrictive properties. First of all, we can mention the so-called. " preparation B-190 " is a means of emergency medical protection with external radiation exposure. Substitute indralin , an imidazole derivative, an agonist of a- adreno - reactive structures of the body, is intended for use in extreme situations accompanied by the threat of exposure in doses of more than 1 Gy to reduce the severity of acute radiation damage to the body. The drug is administered orally at a dose of 0.45 g (3 tablets of 0.15 g) for 10-15 minutes before the intended exposure. The duration of the radioprotector is about 1 hour. It is almost impossible to find. Another effective radioprotector from the imidazoline group is naphthyzine . Introduced in a volume of 1 ml for 3-5 minutes before the intended exposure. It is true that in our pharmacies, banal drops in the nose are more common ... In addition, there is one nuance. The use of radioprotectors for short-term irradiation at doses of less than 1 Gy is impractical because of the absence of a practically significant anti-radiation effect under these conditions.
To protective drugs can be attributed, and drugs that increase the body's resistance to radiation due to the ability for a sufficiently long period to increase the so-called "endogenous background of radiation resistance." «» ( ) ( , , ) «» ( ) «». , ( ) , . . - ( ). — ( ). ( , ). , , . 24 : 15 (75 ).
: ( — , , , — : - «», «», «», «» ..), – . 14-21 2-3 20-30 30 3 . : () . . , , .
,
Zhang JS, Sigdestad CP, Gemmell MA, Grdina DJ Modification of radiation response in mice by fractionated extracts of Panax Ginseng // Radiat.Res.— 1987. — V. 112, № 1. — P. 156—163.
Kim SH, Cho CK, Yoo SY et al. In vivo radioprotective activity of Panax giuseng and diethyldithiocarbanate // In vivo — 1993. — V. 7, № 5. — P. 467—470.
Takeda A., Katoh N., Yonezawa M. Restoration of radiation injury by ginseng 3. Radioprotective effect of thermostable fraction ginseng extract on mice, rats and guinea pigs // J. Radiat. Res. (Tokyo) — 1982. — V. 23, № 2. — P.150—167.
Kim SH, Cho CK, Yoo SY et al. In vivo radioprotective activity of Panax giuseng and diethyldithiocarbanate // In vivo — 1993. — V. 7, № 5. — P. 467—470.
Takeda A., Katoh N., Yonezawa M. Restoration of radiation injury by ginseng 3. Radioprotective effect of thermostable fraction ginseng extract on mice, rats and guinea pigs // J. Radiat. Res. (Tokyo) — 1982. — V. 23, № 2. — P.150—167.
That's all. (
UPD : , Ark_V
№1
What is necessary:
) aliexpress c 0,01

) pH aliexpress

1) . , « » . :
FeCl 3 + K 4 [Fe(CN) 6 ] → KFe[Fe(CN) 6 ] + 3KCl
31,5 /100 25 °C. ( ). 15 E536 100 . (III). . . , . FeCl 3 · 6 2 . 11,5 ( ) 100 . ( , . , 50 °C. .
2) .
( , , — ). 110,5 /100 ( 20 °C). 50 ( 1.5 ), 100 . ( 9%).
K 2 CO 3 + CH 3 COOH → CH 3 COOK + KHCO 3
50 200 . . , 7 . , 300 30 , .. 10% . .
) aliexpress c 0,01

) pH aliexpress

1) . , « » . :
FeCl 3 + K 4 [Fe(CN) 6 ] → KFe[Fe(CN) 6 ] + 3KCl
31,5 /100 25 °C. ( ). 15 E536 100 . (III). . . , . FeCl 3 · 6 2 . 11,5 ( ) 100 . ( , . , 50 °C. .
2) .
( , , — ). 110,5 /100 ( 20 °C). 50 ( 1.5 ), 100 . ( 9%).
K 2 CO 3 + CH 3 COOH → CH 3 COOK + KHCO 3
50 200 . . , 7 . , 300 30 , .. 10% . .
NB / — FB . , .
.. , . – . , 2004.
.. . – , 1989.
.. , . – .,1992.
. .. . – ., ,1998.
.. . : . – , 2003.
.. . – , 1989.
.. , . – .,1992.
. .. . – ., ,1998.
.. . : . – , 2003.
Source: https://habr.com/ru/post/454766/
All Articles
