Opus about His Majesty Clay. Part three - polyurethane vs cosmic cold
Dedicated to all water tourists, fishermen and sailors who succeeded or failed (but I hope it will be possible after reading the article) to seal holes in their PVC vessels, because it is not Desmocol’s single ...
Polyurethane entered my life in early childhood, when I ardently argued with the mother that it was more profitable to buy expensive Belkelme sneakers with a polyurethane sole, and not Lidian ones with unintelligible plastic that broke out (later, by the way, “Lidic” were corrected) simply because “ polyurethane - the eternal. In principle, the way it was, sneakers wore out to rot, and the sole, the sole looked like on the day of purchase. The second “discovery” of this important class of polymers for me took place when I became acquainted with water tourism and such a thing as a hole in the “skin” of PVC. I still remember the commandment of the coach, ps Vyacheslav Antonovich Bazhansky - "Desmokol-created for kayaks, like a bird to the sky."
So, friends, today your next, third article from the “glue” series. It is dedicated to polyurethane . If you want to find out how to clean the mounting foam, how to glue up the pierced fishing pvc boat tightly and what kind of sealant maintains its elasticity at liquid nitrogen temperature - go under the cut, everything is there!
')

In the last article about superglue-cyanoacrylate, I gave a curious picture. Now in order not to duplicate, I hide it under a spoiler.
From this picture it is clear that polyurethanes (PU) absolutely deservedly occupy the first place among all other adhesives. Understanding the reasons will help the plate below, describing the advantages and disadvantages of this type of adhesives.
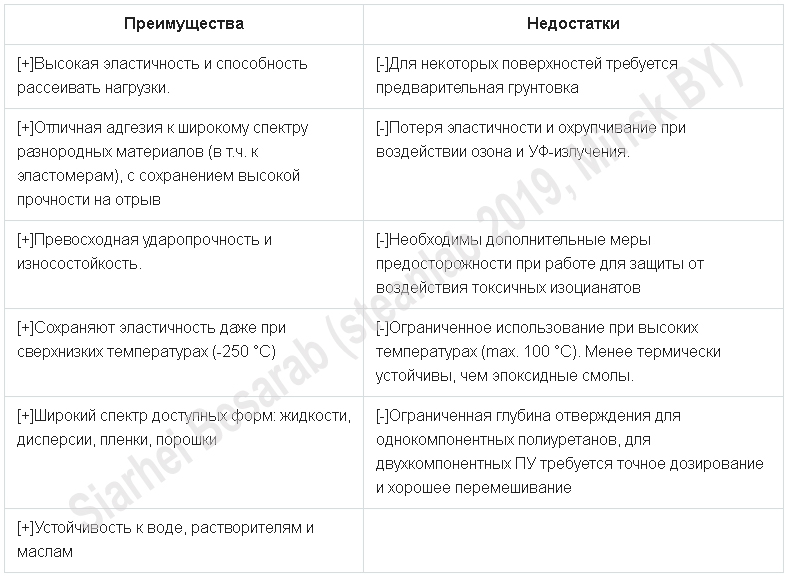
Some disadvantages and merits will give a more detailed interpretation below, but for now, traditionally, facts from history.
Polyurethanes were first synthesized in Germany by Otto Bayer and his staff at IG Farbenindustrie in the late 1930s (the first patents date back to 1937).

The most talented chemist, by the way. "Father of polyurethane" and the founder of the now famous chemical giant Bayer AG. His memory was immortalized in Germany with the annual Otto Baier Prize for outstanding scientific discoveries in the field of chemistry and biochemistry.
The first "children of Uncle Otto" were obtained by reacting an aliphatic diisocyanate with an aliphatic diamine or diol . In contrast to the severe fate of cyanoacrylate, these materials almost immediately found commercial use and began to be sold under the trade names Irgamid U for plastics and Perlon U for synthetic fibers and bristles. After some time, it was found that isocyanates can be used to bond rubber to metal, which, in turn, led to a surge in interest in the synthesis of urethane adhesives based on polyether diols. The first commercial product of this type was produced under the brand name Polystal.
That just did not glue the invented substances - from life rafts to equipment tanks. In the early 1950s, urethane prepolymers were first used to combine leather, wood, fabric, and rubber composites. A few years later, one of the first two-component urethane adhesives was proposed for use as an adhesive for metal-to-metal compounds. In 1957, the first thermoplastic polyurethane appeared, which was used as a hot melt adhesive (adhesive tapes), which was used to glue sheet metal containers. New thermoplastic polyurethane adhesives began to appear in the period 1958-1959. In the early 1960s, BFGoodrich tire companies developed thermoplastic polyester polyurethanes, which glued leather and vinyl. In 1968, Goodyear introduced the first structural adhesive for fiberglass used for the hood of trucks. Pressure-sensitive polyurethanes began to appear in the early 1970s. Under the same Goodyear brand, by 1978, advanced two-component water-based automotive adhesives and adhesives appeared on the market. In 1984, Bostik developed reactive hot melt adhesives. Well, then you already know ... Further, the evolution of adhesives and the latest trends in this area I have already described in one of my previous articles.
In general, almost a hundred years of detailed study and continuous development of new compositions led to the fact that today polyurethanes are one of the most well-known universal polymer systems. This is confirmed by the variety of products that are manufactured using PU - elastic fibers and wear-resistant coatings, all kinds of foams, shock absorbers, adhesives and tens and hundreds more products.

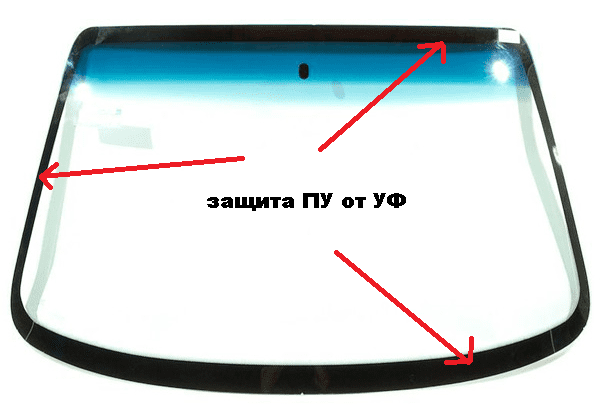
It can be safely argued that an extensive niche of polyurethane adhesives and sealants in the automotive industry (constructional sealants, adhesives for plastics and fillers), the construction field (wood connection, production of chipboard and laminates, gluing large areas of metal plates and composites, installation of glazing systems, high-strength floors, building sealants), shipbuilding (assembly adhesives, sealants) and the shoe industry (main adhesives) - is firmly and permanently occupied. For example, I wouldn’t be mistaken if I say that all windshields of cars are kept on polyurethane. By the way, a black stripe along the edge of the glass is not so much an aesthetic element as protection for polyurethane glue from ultraviolet radiation.
Here you can also recall such a thing quite far from the same foam as a thing like Lycra , it is also elastane, it’s also spandex - a synthetic fabric with unique elasticity (I immediately recall the advertisement “blah blah blah ... tights with lycra”). By the way, these fibers look equally nice under polarized light:

Whatever one may say, it is impossible to show long pictures, anyway, in order to explain the reasons for many physical properties of polymers, one will have to descend to the very basics, to the chemistry of high-molecular compounds. What we do below and do.
Polyurethanes are polymers containing the -NHCOO- urethane groups in the polymer chain. They are most often obtained by the interaction of di- or tripolyisocyanate with a polyhydric alcohol ( polyol ). Since polyurethanes contain two types of monomers that polymerize one by one, they are classified as alternating copolymers. Both the isocyanates and the polyols (polyhydric alcohols) used to make polyurethanes contain an average of two or more functional groups per molecule. The diagram below shows the main “building blocks” of any polyurethane.

In fairness it should be noted that often in "commercial" polyurethanes other "extraneous" functional groups (ether, ester, amide or urea) are present in quantities much larger than the number of the same name, urethane groups.

By the way, a small remark. Polyurethanes are often simply called “urethanes” and should not be confused with compounds such as ethyl carbamate (carbamic acid ethyl ester), which organic chemists often call urethane. Our "saber" urethanes have nothing to do with ethyl carbamate. In general, when they say "urethane glue", usually mean adhesive polymer, obtained by the methods of the chemistry of isocyanates using reactions of isocyanates with active hydrogen compounds. But, be they wrong, the isocyanate reactions do not always lead to the formation of urethane bonds, just as there are ways to obtain compounds with urethane bonds without the use of isocyanates. Therefore, in order to avoid confusion, it is believed that a polyurethane adhesive is an adhesive that, for the polymerization and binding of materials, uses reactions involving the isocyanate group (—N = C = O, NCO).
As will be shown below, the properties of polyurethane largely depend on the types of isocyanates and polyols used to make it. Long flexible segments introduced by the polyol give a soft, elastic polymer. Large amounts of crosslinks give rigid polymers. Long chains and weak crosslinking gives a polymer that is very elastic, short chains with a lot of crosslinks give a hard polymer. In some respects, a piece of polyurethane can be considered as one giant molecule. One of the consequences of this is that typical polyurethanes do not soften and do not melt when heated, they are thermosetting. This is quite an important property for glue, which claims to be the pedestal of the world of structural adhesives.
The correct structural adhesive (adhesive) must necessarily have molecules with three functional groups in order to form a three-dimensional structure with covalent bonds (otherwise a conventional thermoplastic would result). Therefore, the concentration of such molecules is decisive in the formation of the “working” structure of the adhesive (= the number of cross-links, which are lower).
If you recall the label that the better to glue, which I showed in the first article:

You can see that polyurethane adhesives are best suited for bonding between elastomers / fabrics and composites / polymers with glass and ceramics / wood / metal. Those. The main use of polyurethanes is to bind plastics that are difficult to bind, usually with a dissimilar material or with metals. The following are the reasons why PUs are excellent adhesives:
1. PU effectively wets the surface of most materials (although for materials with low surface energy, we again recall polyethylene or polypropylene, to increase wetting it is necessary to use primers or adhesives with a special additive-wetting agent),
2. PU easily form hydrogen bonds with the surface of the material,
3. The small molecular size of the PU allows them to penetrate into the porous and layered materials, to wet the fibers
4. PU form covalent bonds with materials that have active hydrogen atoms (polar surface). The mechanism of formation of such a covalent bond of a polyurethane adhesive with a polar surface is shown in the picture:

Polyurethanes spatially represent a segmented polymer consisting of soft segments and hard segments (random or (AB) block polymer). The soft segment consists of a long chain polyol, for example, polyester or polyetherdiol. The hard segment is formed from a polyisocyanate and a short chain extension from polyol or diamine.
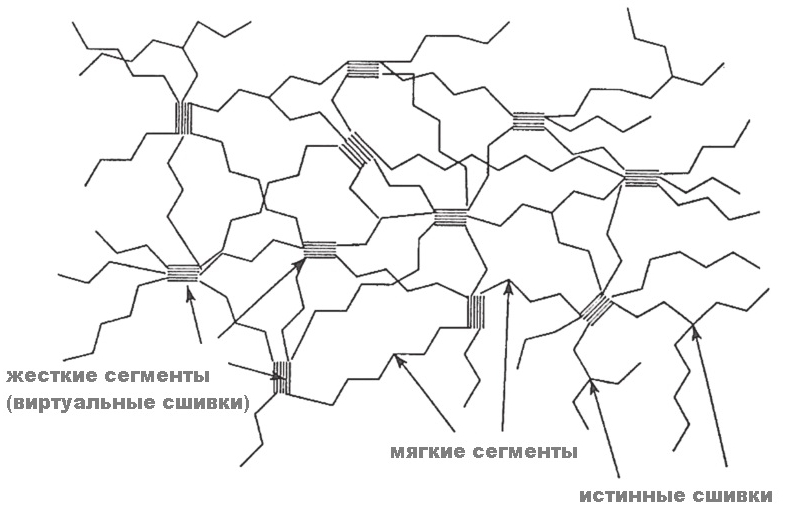
Due to the thermodynamic incompatibility of the two segments, phase separation occurs, leading to the formation of domains or microdomains within the polymer. It is the presence of these microdomains that forms the unique viscoelastic behavior inherent only to polyurethanes. It is precisely the two-phase morphology of PU that is due to impact strength and a wide range of physical properties. Hard domains enhance the polymer and act as crosslinking centers. Finally, virtual stitches (pseudo-stitches) are formed. Due to this structure, unusual material properties are formed (= there are, unlike most polymers, two temperature transitions, the first low-temperature (-20 ° C) due to the glass transition of the soft segment and the second, high-temperature (> 80 ° C) associated with thermal dissociation of hard segment microdomains). Those. microdomains are what polyurethane makes polyurethane (and there are no hydrogen bonds there, although they often tried to ascribe the laurels to the creators of the unique properties of PUs). Uniqueness is visible from the plate, -240 ° C is practically the temperature of absolute zero temperature (−273.15 ° C), and here some material can also afford to stretch ...

I voiced the definition of structural glue; now I’m also voicing the fact that polyurethanes actively compete for the title of main structural glue with epoxides and acrylics. The figure shows a rough comparison of the main structural adhesives in terms of tensile elongation / shear modulus / shear strength.
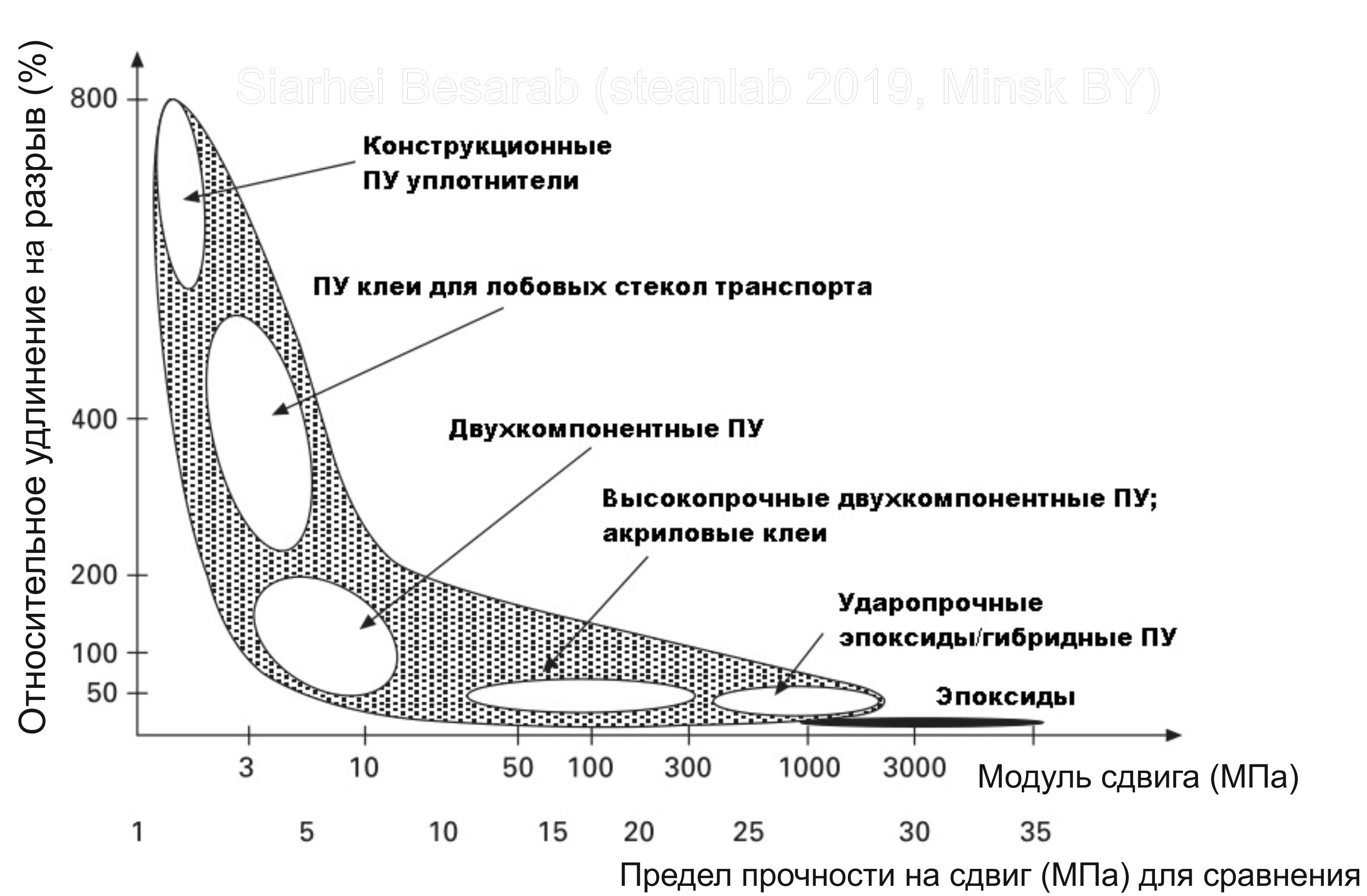
It is these properties of glue, in fact, are the key to understanding the applicability in terms of specific technical specifications. The shear modulus characterizes the stiffness of the bond glued together, the relative elongation at break shows whether it is possible to connect different in terms of thermal elongation of the substrate with each other with an elongation of the bonding line. Shear strength increases linearly with shear modulus. As follows from the graph, polyurethanes "ripen everywhere" and therefore are the most versatile adhesive. An important advantage is that their properties can be regulated very widely in a wide range - from very soft and elastic, rubbery to very hard and hard. The main goal in the development of a good structural adhesive is to achieve the highest possible modulus of shear while maintaining good elongation at break (= if there was an ideal structural adhesive - it would be in the upper right corner of the graph while it was empty ...).
Now it's time to go to the classification of the world of polyurethanes. The following table is intended to help this:

The new concept is the so-called. "Blocked isocyanates". Isocyanate blocking or masking is the reaction of isocyanate groups with a compound that prevents the isocyanate from interacting with the polar surface at room temperature, but will allow this reaction to occur at elevated temperatures. In addition, blocking allows to solve the problem of hypersensitivity to moisture and create aqueous dispersions or dispersions of isocyanate in a polyhydric alcohol. Thanks to these capabilities, dozens of compositions of adhesives for coating / laminating fabrics, adhesives for tire cords, etc., have been developed, which is impossible when using traditional components.
Polyurethane adhesives are divided into two main groups in accordance with different curing mechanisms:
1. One-component adhesives are usually cured by moisture.
2. Two-component adhesives are mixtures of resins and hardeners.
One-component polyurethane adhesives depend on the humidity of the air and emit isocyanates in various quantities during the curing cycle (by the way, when using construction foam, I recommend walls / crevices, etc., to moisten abundantly with water, well, or alcohol, if not sorry). Curing takes place rather slowly, the glue sets in 20-30 minutes and completely cures in 3-7 days depending on the composition. The isocyanates, though not lethal in reasonable quantities, are nonetheless a fairly strong irritant agent / skin sensitizer , so the standard wish that is present in all my recent polymer articles is an “ insulating mask with a cartridge for“ gases / vapors ”and / or ventilation . " And if in the case of gluing a small object to get a dangerous dose of isocyanate is difficult, in the case of foaming thecar’s walls in someone else’s garage , it is quite possible. The fact that the problem exists, says the concern of the EPA (US Environmental Protection Agency), which issued a special card for this brand with safety tips for thermal insulation with polyurethane spray foam (SPF).

As for the most common components of this group of adhesives, the following can be mentioned:
Hydroxypolyurethanes are thermoplastics with a content of hydroxyl groups of 0.5-1.0% and are obtained by reacting diphenymethane-4-4 -diisocyanate (MDI) with polyetherdiols. Thermoplastic polyurethanes are obtained when hydroxylated polyurethanes are mixed with polyisocyanates. Curing occurs at room temperature. The process with the participation of such glue can be called differently: thermal bonding, high-temperature vulcanization, reactionary gluing, melt-bonding, etc. The difference from polymers that are used in traditional glue guns is that TPU is melted only for ease of application, the final properties of the glue line are formed in the process of classical moisture curing of air at room temperature. Hot-melt glue from the gun immediately forms a glue seam after cooling. When emulsifying linear PU in water, polyurethane dispersion adhesives are obtained, which are actively used in the packaging and textile industry.
Unlike single-component, two-component polyurethane adhesives consist of low molecular weight polyisocyanates or prepolymers, cured by low molecular weight polyols or polyamines. Despite the change in the curing method, these PUs also sin with the release of isocyanates, but in smaller quantities. A distinctive feature is the reduced time required for acquiring maximum strength with glue seam. It is two-component PU used in the automotive industry for bonding metals with plastics, for foaming textile coatings, for gluing PVC film with wood in the furniture industry, etc.
In general, both one- and two-component PUs cure well at room temperature (with the exception of the already mentioned melting melts), but it is worth noting that heating the joint and seam accelerates the cross-linking of the polymer. More visibility to change the strength of the adhesive joint, depending on the speed of curing of different polyurethanes can give the picture:
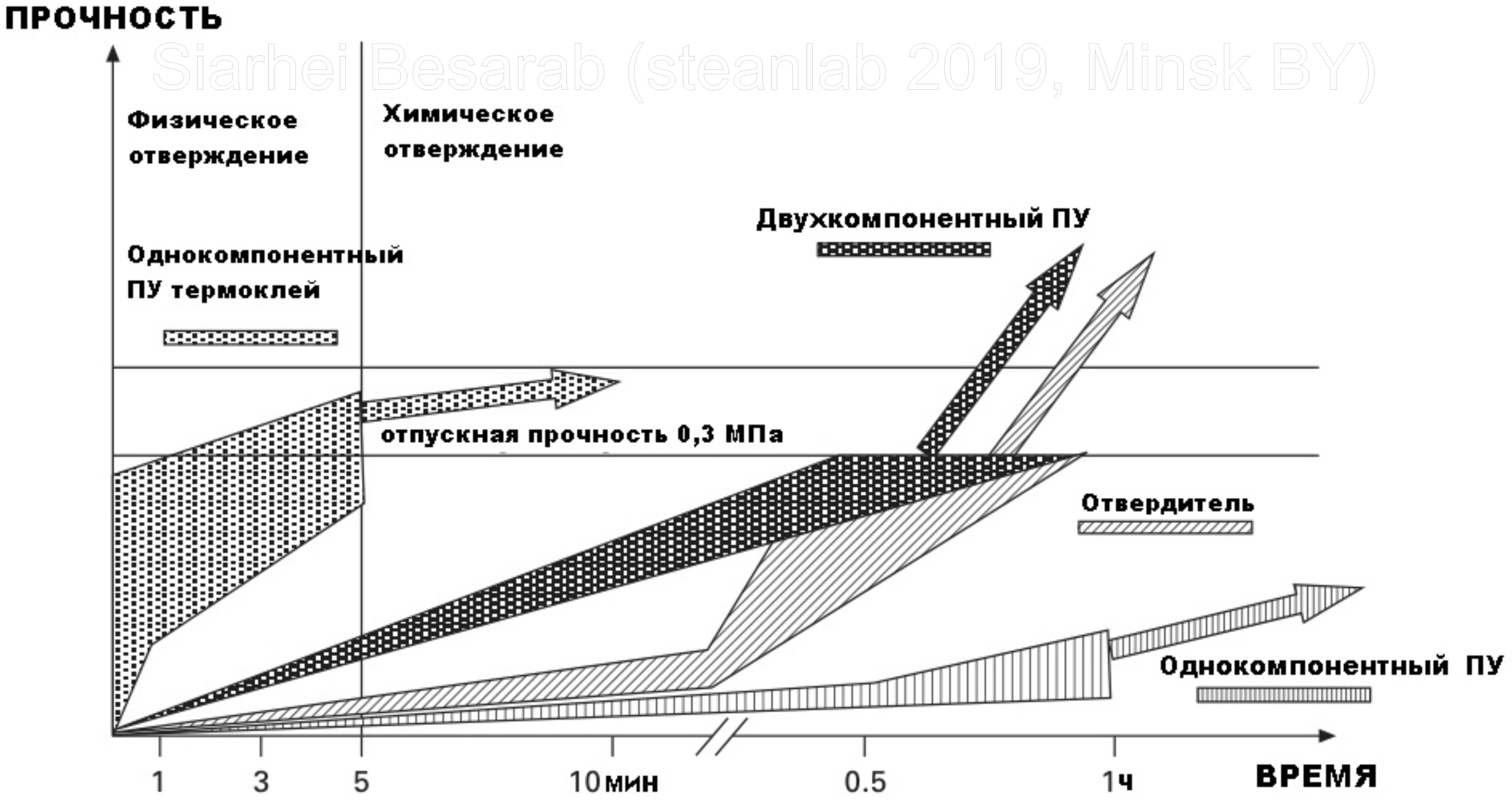
For binding, a figure of 0.3 MPa is given, since this is exactly the level that polymer producers are guided by when developing the composition of the adhesive for windshields. From the graph it is clear that slow curing requires a longer time to fix the objects to achieve the desired strength. Therefore, the glass is heated, thereby reducing the time to set the required strength characteristics to 1 hour instead of 24 hours. True, not everything is as simple as it would seem, because if the glue cools too quickly, it may eventually lead to detachment (an increase in cohesion occurs faster than the return of adhesive properties) and cause cracking of the glue under varying loads from air currents.
Separately - a few words about the preparation of the surface before gluing. The main purpose of surface treatment is to remove any weak surface boundary layer on the substrate and / or use primers. Mechanical treatment (“wipe sandpaper”) and degreasing the surface with a solvent is traditional for PU methods. Isocyanates themselves which often react with polar groups on the surface and promote binding, or silanes, are often used as primers.Silanes are commonly used for glass (maximum efficiency — the alkoxysilane of the primer is bound to the silanol groups on the glass surface, and the amino, mercapto or epoxy group of the same silane is attached to the isocyanate group of glue), fibrous composites, mineral fillers and cementing surfaces. Epoxy silanes are also added to polyurethane adhesives to improve water resistance. Primers based on organic metal complexes are also known - organotitanates, organozirconates and some chromium complexes.
At the end of the story - a few thematic spoilers with the "practical life".
Polyurethane entered my life in early childhood, when I ardently argued with the mother that it was more profitable to buy expensive Belkelme sneakers with a polyurethane sole, and not Lidian ones with unintelligible plastic that broke out (later, by the way, “Lidic” were corrected) simply because “ polyurethane - the eternal. In principle, the way it was, sneakers wore out to rot, and the sole, the sole looked like on the day of purchase. The second “discovery” of this important class of polymers for me took place when I became acquainted with water tourism and such a thing as a hole in the “skin” of PVC. I still remember the commandment of the coach, ps Vyacheslav Antonovich Bazhansky - "Desmokol-created for kayaks, like a bird to the sky."
So, friends, today your next, third article from the “glue” series. It is dedicated to polyurethane . If you want to find out how to clean the mounting foam, how to glue up the pierced fishing pvc boat tightly and what kind of sealant maintains its elasticity at liquid nitrogen temperature - go under the cut, everything is there!
')

In the last article about superglue-cyanoacrylate, I gave a curious picture. Now in order not to duplicate, I hide it under a spoiler.
Glue Market Distribution
From this picture it is clear that polyurethanes (PU) absolutely deservedly occupy the first place among all other adhesives. Understanding the reasons will help the plate below, describing the advantages and disadvantages of this type of adhesives.

Some disadvantages and merits will give a more detailed interpretation below, but for now, traditionally, facts from history.
Polyurethanes were first synthesized in Germany by Otto Bayer and his staff at IG Farbenindustrie in the late 1930s (the first patents date back to 1937).

The most talented chemist, by the way. "Father of polyurethane" and the founder of the now famous chemical giant Bayer AG. His memory was immortalized in Germany with the annual Otto Baier Prize for outstanding scientific discoveries in the field of chemistry and biochemistry.
The first "children of Uncle Otto" were obtained by reacting an aliphatic diisocyanate with an aliphatic diamine or diol . In contrast to the severe fate of cyanoacrylate, these materials almost immediately found commercial use and began to be sold under the trade names Irgamid U for plastics and Perlon U for synthetic fibers and bristles. After some time, it was found that isocyanates can be used to bond rubber to metal, which, in turn, led to a surge in interest in the synthesis of urethane adhesives based on polyether diols. The first commercial product of this type was produced under the brand name Polystal.
That just did not glue the invented substances - from life rafts to equipment tanks. In the early 1950s, urethane prepolymers were first used to combine leather, wood, fabric, and rubber composites. A few years later, one of the first two-component urethane adhesives was proposed for use as an adhesive for metal-to-metal compounds. In 1957, the first thermoplastic polyurethane appeared, which was used as a hot melt adhesive (adhesive tapes), which was used to glue sheet metal containers. New thermoplastic polyurethane adhesives began to appear in the period 1958-1959. In the early 1960s, BFGoodrich tire companies developed thermoplastic polyester polyurethanes, which glued leather and vinyl. In 1968, Goodyear introduced the first structural adhesive for fiberglass used for the hood of trucks. Pressure-sensitive polyurethanes began to appear in the early 1970s. Under the same Goodyear brand, by 1978, advanced two-component water-based automotive adhesives and adhesives appeared on the market. In 1984, Bostik developed reactive hot melt adhesives. Well, then you already know ... Further, the evolution of adhesives and the latest trends in this area I have already described in one of my previous articles.
In general, almost a hundred years of detailed study and continuous development of new compositions led to the fact that today polyurethanes are one of the most well-known universal polymer systems. This is confirmed by the variety of products that are manufactured using PU - elastic fibers and wear-resistant coatings, all kinds of foams, shock absorbers, adhesives and tens and hundreds more products.

Polyurethane foam - the main instrument of the village Hohmach

It can be safely argued that an extensive niche of polyurethane adhesives and sealants in the automotive industry (constructional sealants, adhesives for plastics and fillers), the construction field (wood connection, production of chipboard and laminates, gluing large areas of metal plates and composites, installation of glazing systems, high-strength floors, building sealants), shipbuilding (assembly adhesives, sealants) and the shoe industry (main adhesives) - is firmly and permanently occupied. For example, I wouldn’t be mistaken if I say that all windshields of cars are kept on polyurethane. By the way, a black stripe along the edge of the glass is not so much an aesthetic element as protection for polyurethane glue from ultraviolet radiation.

Here you can also recall such a thing quite far from the same foam as a thing like Lycra , it is also elastane, it’s also spandex - a synthetic fabric with unique elasticity (I immediately recall the advertisement “blah blah blah ... tights with lycra”). By the way, these fibers look equally nice under polarized light:

And so it looks in public. as opposed to tights

The reason for such popularity?
Whatever one may say, it is impossible to show long pictures, anyway, in order to explain the reasons for many physical properties of polymers, one will have to descend to the very basics, to the chemistry of high-molecular compounds. What we do below and do.
Polyurethanes are polymers containing the -NHCOO- urethane groups in the polymer chain. They are most often obtained by the interaction of di- or tripolyisocyanate with a polyhydric alcohol ( polyol ). Since polyurethanes contain two types of monomers that polymerize one by one, they are classified as alternating copolymers. Both the isocyanates and the polyols (polyhydric alcohols) used to make polyurethanes contain an average of two or more functional groups per molecule. The diagram below shows the main “building blocks” of any polyurethane.

In fairness it should be noted that often in "commercial" polyurethanes other "extraneous" functional groups (ether, ester, amide or urea) are present in quantities much larger than the number of the same name, urethane groups.

By the way, a small remark. Polyurethanes are often simply called “urethanes” and should not be confused with compounds such as ethyl carbamate (carbamic acid ethyl ester), which organic chemists often call urethane. Our "saber" urethanes have nothing to do with ethyl carbamate. In general, when they say "urethane glue", usually mean adhesive polymer, obtained by the methods of the chemistry of isocyanates using reactions of isocyanates with active hydrogen compounds. But, be they wrong, the isocyanate reactions do not always lead to the formation of urethane bonds, just as there are ways to obtain compounds with urethane bonds without the use of isocyanates. Therefore, in order to avoid confusion, it is believed that a polyurethane adhesive is an adhesive that, for the polymerization and binding of materials, uses reactions involving the isocyanate group (—N = C = O, NCO).
As will be shown below, the properties of polyurethane largely depend on the types of isocyanates and polyols used to make it. Long flexible segments introduced by the polyol give a soft, elastic polymer. Large amounts of crosslinks give rigid polymers. Long chains and weak crosslinking gives a polymer that is very elastic, short chains with a lot of crosslinks give a hard polymer. In some respects, a piece of polyurethane can be considered as one giant molecule. One of the consequences of this is that typical polyurethanes do not soften and do not melt when heated, they are thermosetting. This is quite an important property for glue, which claims to be the pedestal of the world of structural adhesives.
Structural adhesive is an adhesive substance used to transfer the necessary loads between adhesive surfaces in complex structures exposed to the operating environment.
The correct structural adhesive (adhesive) must necessarily have molecules with three functional groups in order to form a three-dimensional structure with covalent bonds (otherwise a conventional thermoplastic would result). Therefore, the concentration of such molecules is decisive in the formation of the “working” structure of the adhesive (= the number of cross-links, which are lower).
If you recall the label that the better to glue, which I showed in the first article:

You can see that polyurethane adhesives are best suited for bonding between elastomers / fabrics and composites / polymers with glass and ceramics / wood / metal. Those. The main use of polyurethanes is to bind plastics that are difficult to bind, usually with a dissimilar material or with metals. The following are the reasons why PUs are excellent adhesives:
1. PU effectively wets the surface of most materials (although for materials with low surface energy, we again recall polyethylene or polypropylene, to increase wetting it is necessary to use primers or adhesives with a special additive-wetting agent),
2. PU easily form hydrogen bonds with the surface of the material,
3. The small molecular size of the PU allows them to penetrate into the porous and layered materials, to wet the fibers
4. PU form covalent bonds with materials that have active hydrogen atoms (polar surface). The mechanism of formation of such a covalent bond of a polyurethane adhesive with a polar surface is shown in the picture:

Polyurethanes spatially represent a segmented polymer consisting of soft segments and hard segments (random or (AB) block polymer). The soft segment consists of a long chain polyol, for example, polyester or polyetherdiol. The hard segment is formed from a polyisocyanate and a short chain extension from polyol or diamine.

Due to the thermodynamic incompatibility of the two segments, phase separation occurs, leading to the formation of domains or microdomains within the polymer. It is the presence of these microdomains that forms the unique viscoelastic behavior inherent only to polyurethanes. It is precisely the two-phase morphology of PU that is due to impact strength and a wide range of physical properties. Hard domains enhance the polymer and act as crosslinking centers. Finally, virtual stitches (pseudo-stitches) are formed. Due to this structure, unusual material properties are formed (= there are, unlike most polymers, two temperature transitions, the first low-temperature (-20 ° C) due to the glass transition of the soft segment and the second, high-temperature (> 80 ° C) associated with thermal dissociation of hard segment microdomains). Those. microdomains are what polyurethane makes polyurethane (and there are no hydrogen bonds there, although they often tried to ascribe the laurels to the creators of the unique properties of PUs). Uniqueness is visible from the plate, -240 ° C is practically the temperature of absolute zero temperature (−273.15 ° C), and here some material can also afford to stretch ...

I voiced the definition of structural glue; now I’m also voicing the fact that polyurethanes actively compete for the title of main structural glue with epoxides and acrylics. The figure shows a rough comparison of the main structural adhesives in terms of tensile elongation / shear modulus / shear strength.

It is these properties of glue, in fact, are the key to understanding the applicability in terms of specific technical specifications. The shear modulus characterizes the stiffness of the bond glued together, the relative elongation at break shows whether it is possible to connect different in terms of thermal elongation of the substrate with each other with an elongation of the bonding line. Shear strength increases linearly with shear modulus. As follows from the graph, polyurethanes "ripen everywhere" and therefore are the most versatile adhesive. An important advantage is that their properties can be regulated very widely in a wide range - from very soft and elastic, rubbery to very hard and hard. The main goal in the development of a good structural adhesive is to achieve the highest possible modulus of shear while maintaining good elongation at break (= if there was an ideal structural adhesive - it would be in the upper right corner of the graph while it was empty ...).
Now it's time to go to the classification of the world of polyurethanes. The following table is intended to help this:

The new concept is the so-called. "Blocked isocyanates". Isocyanate blocking or masking is the reaction of isocyanate groups with a compound that prevents the isocyanate from interacting with the polar surface at room temperature, but will allow this reaction to occur at elevated temperatures. In addition, blocking allows to solve the problem of hypersensitivity to moisture and create aqueous dispersions or dispersions of isocyanate in a polyhydric alcohol. Thanks to these capabilities, dozens of compositions of adhesives for coating / laminating fabrics, adhesives for tire cords, etc., have been developed, which is impossible when using traditional components.
One and two component adhesives
Polyurethane adhesives are divided into two main groups in accordance with different curing mechanisms:
1. One-component adhesives are usually cured by moisture.
2. Two-component adhesives are mixtures of resins and hardeners.
One-component polyurethane adhesives depend on the humidity of the air and emit isocyanates in various quantities during the curing cycle (by the way, when using construction foam, I recommend walls / crevices, etc., to moisten abundantly with water, well, or alcohol, if not sorry). Curing takes place rather slowly, the glue sets in 20-30 minutes and completely cures in 3-7 days depending on the composition. The isocyanates, though not lethal in reasonable quantities, are nonetheless a fairly strong irritant agent / skin sensitizer , so the standard wish that is present in all my recent polymer articles is an “ insulating mask with a cartridge for“ gases / vapors ”and / or ventilation . " And if in the case of gluing a small object to get a dangerous dose of isocyanate is difficult, in the case of foaming the

As for the most common components of this group of adhesives, the following can be mentioned:
Hydroxypolyurethanes are thermoplastics with a content of hydroxyl groups of 0.5-1.0% and are obtained by reacting diphenymethane-4-4 -diisocyanate (MDI) with polyetherdiols. Thermoplastic polyurethanes are obtained when hydroxylated polyurethanes are mixed with polyisocyanates. Curing occurs at room temperature. The process with the participation of such glue can be called differently: thermal bonding, high-temperature vulcanization, reactionary gluing, melt-bonding, etc. The difference from polymers that are used in traditional glue guns is that TPU is melted only for ease of application, the final properties of the glue line are formed in the process of classical moisture curing of air at room temperature. Hot-melt glue from the gun immediately forms a glue seam after cooling. When emulsifying linear PU in water, polyurethane dispersion adhesives are obtained, which are actively used in the packaging and textile industry.
Unlike single-component, two-component polyurethane adhesives consist of low molecular weight polyisocyanates or prepolymers, cured by low molecular weight polyols or polyamines. Despite the change in the curing method, these PUs also sin with the release of isocyanates, but in smaller quantities. A distinctive feature is the reduced time required for acquiring maximum strength with glue seam. It is two-component PU used in the automotive industry for bonding metals with plastics, for foaming textile coatings, for gluing PVC film with wood in the furniture industry, etc.
In general, both one- and two-component PUs cure well at room temperature (with the exception of the already mentioned melting melts), but it is worth noting that heating the joint and seam accelerates the cross-linking of the polymer. More visibility to change the strength of the adhesive joint, depending on the speed of curing of different polyurethanes can give the picture:

For binding, a figure of 0.3 MPa is given, since this is exactly the level that polymer producers are guided by when developing the composition of the adhesive for windshields. From the graph it is clear that slow curing requires a longer time to fix the objects to achieve the desired strength. Therefore, the glass is heated, thereby reducing the time to set the required strength characteristics to 1 hour instead of 24 hours. True, not everything is as simple as it would seem, because if the glue cools too quickly, it may eventually lead to detachment (an increase in cohesion occurs faster than the return of adhesive properties) and cause cracking of the glue under varying loads from air currents.
Separately - a few words about the preparation of the surface before gluing. The main purpose of surface treatment is to remove any weak surface boundary layer on the substrate and / or use primers. Mechanical treatment (“wipe sandpaper”) and degreasing the surface with a solvent is traditional for PU methods. Isocyanates themselves which often react with polar groups on the surface and promote binding, or silanes, are often used as primers.Silanes are commonly used for glass (maximum efficiency — the alkoxysilane of the primer is bound to the silanol groups on the glass surface, and the amino, mercapto or epoxy group of the same silane is attached to the isocyanate group of glue), fibrous composites, mineral fillers and cementing surfaces. Epoxy silanes are also added to polyurethane adhesives to improve water resistance. Primers based on organic metal complexes are also known - organotitanates, organozirconates and some chromium complexes.
At the end of the story - a few thematic spoilers with the "practical life".
Practical lesson №1 - Polyurethane foam
Practical lesson number 2, or seryozhiny bags
, ...
, , . , .

— , , . — «», « + », «Desmocol», «», «», «88», « », « », «600», «»,«», «- + -021//-/-022», Cosmofen, «». — , .
.
, ? , /«» - - « , , , ». , , , , , - . - , . -, , , - . (« ») . @ divecasper c . — . , :

or

. , . , , — , « » . , , . , , . , «», « » « » .

— . , ( ), , , . , . - :

( //)

, , . . . / . ( ), — . . 6 .

, .

, . , .
, , . , .

— , , . — «», « + », «Desmocol», «», «», «88», « », « », «600», «»,«», «- + -021//-/-022», Cosmofen, «». — , .
.
, ? , /«» - - « , , , ». , , , , , - . - , . -, , , - . (« ») . @ divecasper c . — . , :

or

. , . , , — , « » . , , . , , . , «», « » « » .

— . , ( ), , , . , . - :

( //)

, , . . . / . ( ), — . . 6 .

, .

, . , .
References
WF Gum (ed.) (1992) Reaction Polymers: Polyurethanes, Epoxies, Unsaturated Polyesters, Phenolics, Special Monomers and Additives; Chemistry, Applications, Markets,Hanser, Munich and Oxford University Press, Oxford.
KN Edwards(ed.) (1981) Urethane Chemistry and Applications, ACS Symposium Series 72, Washington, DC.
J. Saunders and K. Frisch. Polyurethanes: Chemistry and Technology, Pt. 1, Interscience, New York (1963).
ME Kimball. Polyurethane adhesives: Properties and bonding procedures. Adhes. Age 24(6), 21–24 (1981).
D. Dieterich and JN Rieck. Aqueous polyurethane systems and their possible uses. Adhes. Age 21(2), 24–27 (1978).
PE Cranley. Polyurethane adhesives. In: Reaction Polymers, WF Gum, W. Riese and H. Ulrich (Eds.), p. 692, Oxford University Press, Hanser Publishers, New York (1992).
U. Meyer-Westhuis. Polyurethanes: Coatings, Adhesives and Sealants, pp. 20–22, Vincentz Narwork, Hanover, Germany (2003)
www.essentialchemicalindustry.org/polymers/polyurethane.html
AJ Kinloch. Adhesion and Adhesives, pp. 101–170, Chapman & Hall, London (1987). —
LD George. Composition. US patent 2,277,083, assigned to Du Pont (1942)./50.
AF Lewis, LM Zaccardo and AM Schiller. Polyurethane based adhesive systems and laminates prepared therewith. US patent 3,391,054, assigned to American Cyanamid Company (1968).
M. Huang and ER Pohl. Organofunctional silanes for sealants. In: Handbook of Sealant Technology, KL Mittal and A. Pizzi (Eds.), pp. 27–49, CRC Press, Boca Raton, FL (2009).
KN Edwards(ed.) (1981) Urethane Chemistry and Applications, ACS Symposium Series 72, Washington, DC.
J. Saunders and K. Frisch. Polyurethanes: Chemistry and Technology, Pt. 1, Interscience, New York (1963).
ME Kimball. Polyurethane adhesives: Properties and bonding procedures. Adhes. Age 24(6), 21–24 (1981).
D. Dieterich and JN Rieck. Aqueous polyurethane systems and their possible uses. Adhes. Age 21(2), 24–27 (1978).
PE Cranley. Polyurethane adhesives. In: Reaction Polymers, WF Gum, W. Riese and H. Ulrich (Eds.), p. 692, Oxford University Press, Hanser Publishers, New York (1992).
U. Meyer-Westhuis. Polyurethanes: Coatings, Adhesives and Sealants, pp. 20–22, Vincentz Narwork, Hanover, Germany (2003)
www.essentialchemicalindustry.org/polymers/polyurethane.html
AJ Kinloch. Adhesion and Adhesives, pp. 101–170, Chapman & Hall, London (1987). —
LD George. Composition. US patent 2,277,083, assigned to Du Pont (1942)./50.
AF Lewis, LM Zaccardo and AM Schiller. Polyurethane based adhesive systems and laminates prepared therewith. US patent 3,391,054, assigned to American Cyanamid Company (1968).
M. Huang and ER Pohl. Organofunctional silanes for sealants. In: Handbook of Sealant Technology, KL Mittal and A. Pizzi (Eds.), pp. 27–49, CRC Press, Boca Raton, FL (2009).
Source: https://habr.com/ru/post/452506/
All Articles


