Opus about His Majesty Clay. Part Two - Viva, cyanoacrylate! Viva, superglue
As I promised in the pilot "sticky" article - we will consider adhesives gradually. In order not to postpone the matter indefinitely, I decided to present to your attention some facts related to my beloved, I will not be afraid of this word, folk glue - with cyanoacrylate " superglue ". In addition, to the best of my ability, I tried, in the framework of the topic of the article, to highlight all the questions that readers asked in the previous part. So, if you are an active user of superglue - do not miss. The most up-to-date information about "soda + superglue", about why superglue needs to be stored in the refrigerator and is it possible to light cotton wool with superglue than to wash it off? What is it gluing? - all under the cut!

Despite the fact that, according to businesswire.com, the global market for adhesives and glues belongs to epoxides and polyurethanes, I decided to start the story with cyanoacrylate glue. The reasons explained at the beginning of the article :)

First of all, traditionally, a small historical introduction to see the glue ’s long journey to our table .
History of
The first patent application describing cyanoacrylate was filed back in 1942. Employees of the BF Goodrich Company obtained this substance as a result of screening materials that could be useful for the production of optical sights.

Although researchers have seen that a new substance can glue everything with which it contacts, there was no military use for it. The development of collecting dust on the shelves until 1958, when several people from the company Eastman Kodak decided to create a new glue, they called "Eastman 910". The slowness of launching the market was due to the fact that in the 1960s technologies were not yet developed that would allow storing and transporting glue without changing its chemical properties. In addition, the first generation adhesives had increased brittleness of the adhesive joint (which led to cracking and flaking), filled the gaps weakly and had a strong odor. In the 1960s, Eastman Kodak sold the rights to cyanoacrylate to Loctite, which in 1971, through modification of the compounds, overcame all the technical problems and released its own cyanoacrylate line, called “Super Bonder”. Since that time, the super glue triumphant march across the planet began (represented by products from Henkel, Loctite, Eastman and Permabond). By the way, Permabond still releases the cyanoacrylate of the original "910" composition.
What's inside?
Cyanoacrylate adhesives are high-speed single-component adhesives based on alkyl-2-cyanoacrylate monomers. The curing reaction of these adhesives is so fast that they are called high-speed adhesives, or superglue. The uniqueness of superglue is that it quickly and firmly binds to various materials, without requiring heating or prolonged pressing. Traditionally, ethyl cyanoacrylate is the basis for superglue, but methyl (the cheapest adhesives), n-butyl, allyl, methoxyethyl and ethoxyethyl cyanoacrylates are used.
Methyl cyanoacrylate , having a minimum molecular size, shows better adhesion to metals and is sometimes more resistant to solvents. Methyl cyanoacrylate is used for bonding plastic / rubber with metal (+ fixing small adjustment screws, rivets, etc.)
Ethyl cyanoacrylate is the most common of all cyanoacrylates and the most widely used. It is best suited for bonding most plastics and elastomers and has excellent adhesion to polycarbonate, ABS, PVC and butyl rubber.
| Material | Breaking force (MPa) |
|---|---|
| steel-steel | 22.8 |
| aluminum-aluminum | 15.7 |
| butyl rubber-butyl rubber | one |
| polybutadiene-polybutadiene | 0.9 |
| neoprene neoprene | 0.7 |
| styrene-butadiene rubber-phenolic plastic | 0.7 |
| phenolic plastic-phenolic plastic | 6.4 |
| phenolic plastic-aluminum | 6.3 |
| aluminum-polyamide | 6.6 |
| polyamide-polyamide | 4.1 |
| acrylic-acrylic | 5.5 |
| ABS-ABS | 4.9 |
| polystyrene polystyrene | 2.3 |
| polycarbonate-polycarbonate | 6.6 |
| polyester (fiberglass) -polyester (fiberglass) | 4.7 |
Alkoxyethyl cyanoacrylates , in contrast to the two brothers mentioned above, do not have a sharp irritating odor and do not allow the material adjacent to the glue line to become cloudy. However, due to its high molecular weight, these compounds slowly cure and have reduced adhesion to polymers and metals.
The curing reaction of any cyanoacrylate is an anionic polymerization initiated by any weakly alkaline components present on the surface of most materials. Moisture adsorbed on glued surfaces or contained in near-surface layers of a material leads to continuous merging in thin layers (which, together with the influence of biogenic amines, explains the excellent bonding of fingers).
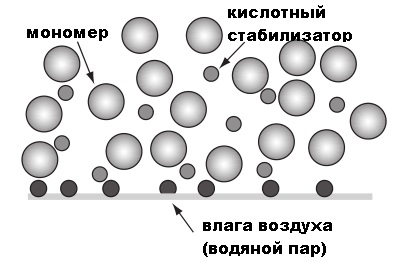
In the picture, the big spheres are the alkyl cyanoacrylate monomer, the small white circles are the acid stabilizer, and the dark spheres represent traces of water on the surface. When the cyanoacrylate comes into contact with OH groups on the surface of the materials to be bonded, the acid stabilizer is neutralized and chains of intertwined adhesive molecules are formed, which bind the surfaces together during the polymerization process.
With the anionic polymerization is associated with the sensational method of "gluing soda" ( habr-post ). This method is suitable for the case when ordinary cyanocrylate (satisfactorily cured only in gaps of 0.05-0.1 mm, which will be mentioned below) must be glued together or spliced any gaps in the material. The seam is successively filled with baking soda, which acts as a filler and alkaline polymerizing agent, and is wetted with cyanoacrylate. As a result, some kind of filled acrylic is obtained. In principle, with the same success it is possible to scrape / nave off the plaster from the walls, it will also provide an alkaline medium and will be able to act as a filler. This method is good for porous materials with which an individual glue works poorly (approx. Without adding special polymerization enhancers, see below). The reaction, depending on the glue, can proceed exothermically (with heat) and sometimes release vapors that are better not to inhale (for toxicity - see the end of the article). By the way, about the "plaster instead of soda." There is an industrially produced glue under the brand name "SupaFix", which uses calcium oxide as the polymerization accelerator (the same quicklime that our builders (RB so precisely, "I guarantee it") are trying to replace cement in plaster). The glue joint is actually stone, so strong that they are even recommended to seal the cracks in the concrete.
The cure rate of cyanoacrylate, if left open on the surface, will be relatively slow (several hours) because there is not enough moisture (although the cyanoacrylate will cure at the interface)
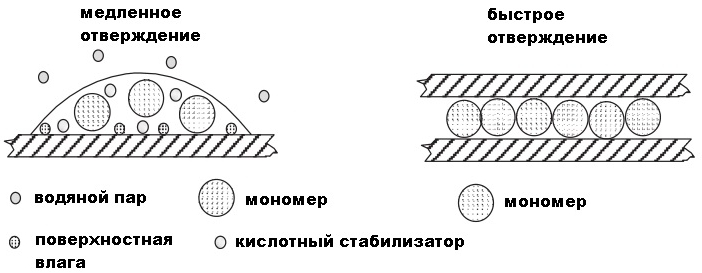
When the adhesive is between two tight-fitting surfaces, moisture appears on both surfaces, and the cyanoacrylate quickly hardens. Therefore, it is the level of humidity and the gap between the surfaces to be glued together that are the main factors affecting the cure rate. The optimal curing conditions for cyanoacrylates are relative humidity from 40% to 60%. Low humidity, for example, 20%, will lead to delayed curing, and high relative humidity, for example 80%, on the one hand polymerizes the glue faster, but on the other, turns the adhesive into a piece of inert polymer before it sticks to the surface to be bonded ( so, the glue line will not work).
Cyanoacrylate adhesives have a short shelf life - about one year from the date of manufacture, if not opened, and one month after opening. This is due, in contrast to solvent evaporation in other types of glue, all with the same sluggish anionic polymerization with the participation of water vapor in the air. Therefore, in the production of glue kept in a dried atmosphere. Therefore, in order to prolong the life of your favorite open tube, observe the following recommendations:
- Store opened cyanoacrylate glue in an airtight container with the maximum possible amount of sachet of drying silica gel. These bags are often put in a package with new shoes, Chinese electronics, etc.
- Plug the hole in the glue supply tube with a needle from a disposable syringe. After using the glue, the residue will clog the needle and block access to moisture. If necessary, it is enough to simply heat the needle with a lighter to open the glue stroke. Polymerized cyanoacrylate - a typical thermoplastic like plexiglass, will float and "open the gateway."
As for tips, keep superglue in the fridge / freezer. This, of course, makes sense, because since the main cause of polymerization is liquid and gaseous water, it is a good option to put the glue where the water is solid - in the freezer with a temperature below 0 ° C (32 ° F; 273 K) - and stop the polymerization reaction completely. But there is one BUT. Sometime the glue will have to be removed from the refrigerator, and moving from cold to heat will cause condensation to form and will give so much water that it does not seem like it. Conclusion - in the cold it makes sense to keep only a sealed tube. If the tube is opened - please ensure smooth step-by-step defrosting (NOFROST there, etc.).
The question may arise, why keep a sealed tube, if the sealed package does not allow moisture to get into the glue. Here the fact is that during storage occurs the so-called. aging of cyanoacrylates, as a result of which they become thicker, increases viscosity, up to turning to stone (+ slow hydrolysis to form cyanoacrylic acid). Cold (below -18 ° C) processes almost completely stop and glue can be stored for an unlimited time. By the way, the thickened, old cyanoacrylate can be diluted with more fresh and liquid glue, similar in composition (= the same brand), and return all the properties.
The gap between the parts to be glued should ideally be less than 0.1 mm, and the smaller the gap, the faster the cure. As a rule, thin gaps give the strongest seams. Although some types of cyanoacrylate allow you to fill gaps up to 0.5 mm, and UV-curable and completely connect gaps up to 5-6 mm.
The strength of the glue line increases with time. As a rule, cyanoacrylates acquire sufficient strength already within the first minute of bonding:
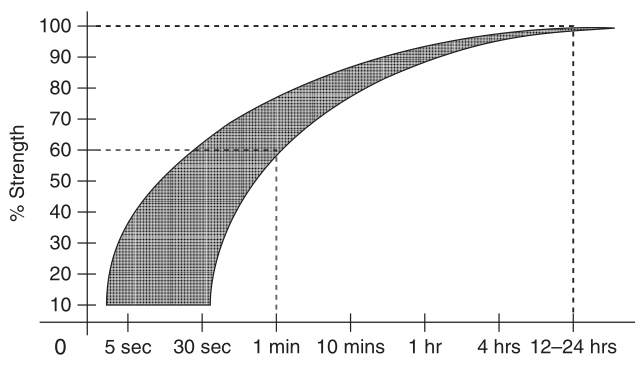
But this process does not stop for the next 24 hours and in some cases can increase strength by half. So, depending on the importance of the parts to be glued, it does not always make sense to immediately use them in everyday life. Cyanoacrylate should not be exposed to physical action during the critical time of polymerization, since the broken glue line can no longer fully acquire its strength.
Cured polyalkyl cyanoacrylate is a pure thermoplastic very similar to plexiglass (PMMA). Individual physical properties depend on the chemical nature of the ether chain. The table summarizes the pros and cons of cyanocrylates.
| Virtues | disadvantages |
|---|---|
| [+] Excellent adhesion to a variety of different materials. | [-] Blurred seam - polymerized monomer vapors appear as white bloom near glue stitching. |
| [+] Very fast one component cure at room temperature. | [-] A long time to build strength in the glue line, stitching parts with a large gap |
| [+] Compound is gaining strength very quickly. | [-] When used with some plastics, cracking occurs when force is applied to the glue line |
| [+] When using primers can be used to connect polyolefins (polyethylene, polypropylene) | [-] Cured glue - thermoplastic with low heat resistance and solvent resistance |
| [-] Thermal and chemical stability is lower than that of other structural adhesives. | |
| [-] High peelability and low impact strength | |
| [-] Relatively high cost | |
| [-]Strong smell | |
| [-] Instantly glued to the skin. |
Cons, as you can see, more. In order to get rid of them, pure cyanoacrylates have to be modified with various additives. Commercial adhesives consist of pure monomer with relatively small amounts of additives, which mainly improve the rheological characteristics of the product (viscosity, etc.). A typical composition for cyanoacrylate glue contains 88% cyanoacrylate monomer, 9% thickener (for example, PMMA, cellulose ethers, rubbers, etc.), 3% rheology modifier (colloidal silica) and 0.02-0.03% acid or radical stabilizer. All functional additives can be divided into two main groups:
- additives that modify the polymerization process
- additives that change the properties of the final polymer.
The modifiers of the polymerization process include stabilizers. Their duty is to ensure a balance between the stability (durability) of the adhesive and the speed of curing. For this purpose, substances inhibiting polymerization are used, most often acidic compounds (in concentrations from 5 to 100 ppm ): SO 2 , sulfamides, SO 3 , sulfonic acids, sulfones, cation exchange resins, boric acid chelates. Free radical polymerization inhibitors like hydroquinone or sterically hindered phenols can also be used.
In contrast to the action of stabilizers, the goal of the so-called. accelerators - an increase in the rate of polymerization. One of the drawbacks of the first generation cyanoacrylate adhesives was that these adhesives could not glue porous materials like paper, wood / cork and leather. The reason for this was that the low viscosity adhesive was absorbed by the porous substrate before it was polymerized. Moreover, the simple thickening of the glue did not give any effect. As a result, a solution was found - substances that are capable of binding alkali metal cations. Interestingly, the mechanism by which accelerators function is unclear, but practical effectiveness has been proven in practice. Examples of compounds used as accelerators are crown ethers , polyalkylene oxides and calixarenes .
In addition to porous materials that absorb glue before it dries, there are materials that have the acidic nature of the surface (for example, many fabrics). Because of this, curing can take a week or even never take place. In this case, accelerators based on crown ethers are also used. The sappience reader also mentioned the so-called. Foam-safe-cyanoacrylate which are used in aircraft modeling and do not damage foam polystyrene (polystyrene foam, styrofoam, EPS, XPS), but also cost much more expensive than the classic superglue. Here, the principle of operation of additives is similar to that described above for fabrics and porous materials (the principle “to polymerize, it is impossible to destroy foam” works - by analogy with the known one, it is impossible to pardon).

Most of the second group of additives that change the properties of the final polymer, allows to obtain cyanoacrylate adhesives with special functional properties. A few words about each in order of priority. About the "surface insensitive" adhesives I have already said. Standard ethyl cyanoacrylate adhesives based on ethyl 2-cyanoacrylate (maybe methyl- and blah blah blah), these are adhesives, which as functional additives contain only thickener (one of the previously described). The remaining additives in the majority solve the individual deficiencies of cyanoacrylates sounded above.
Disadvantage: low toughness. The solution to this problem allows you to create high-strength (impact resistant) cyanoacrylate adhesives, which have a significantly increased impact strength (and low peelability) due to the inclusion of elastomers (ABS or MBS ) in the adhesive composition, which form the separating phases during curing.

Elastomer particles minimize crack propagation due to the fact that the crack reaches a rubber particle, which takes the brunt and dissipates stress. Such adhesives are particularly well suited for bonding rubber to metal. Among the disadvantages of high strength cyanoacrylates, their reduced curing rate can be mentioned. I would like to separately note that there are works in which the use of citric acid as an additive to classical ethyl cyanoacrylate is described, which makes it possible to significantly increase the impact strength of glue when joining metal-metal (for example, aluminum-aluminum).
Disadvantage: brittle glue line. The answer to this flaw was the development of flexible adhesives. These cyanoacrylates have been specifically designed to mount speaker cones to speakers. Flexibility based on aliphatic carboxylic esters of oxalic, tartaric, and citric acids allowed for flexibility. The resulting glue seam does not degrade over time and remains plastic indefinitely. Such adhesives are used for bonding leather products, fabrics, etc. materials (including those having an acidic surface). Among the disadvantages - low temperature resistance (max 75 ° )
: , . , , . - . , , ( ), . (-/-) - . "" — .
: "" (PE,PP, PTFE) .
for metal, surface degreasing and sandblasting; for polymers, solvent cleaning and optional surface polishing; for glass and ceramics, surface cleaning and drying.
, - . ( "" ). (c (SA), (DSA), (DMSA) (DSMA)) . .

: (~) . . — . , jar_ohty "... . , , . , . — . 500 , 80 , 180 , 80-100°C, 15-20 . , ".
— " Tetra-Etch ". (+ ). 10 . … . . .
: . — (<0,2 ). ( /"" aO) . - , ( 365 ). 3 . - 5 6 . , , , .
: . , , — PMMA. , , , 85 ° 100 °, 100 °. , 140 °C. , . , . :
| Polymer | |
|---|---|
| 160 | |
| 138 | |
| - | 90 |
| 130 | |
| 85 | |
| ten |
. , , . 100 ° C. 120 °C . , , , . -2- . , — .
: . , , . , . pH 7. , . , . , , , , (. ), . NB , , , .
fun-...
- , . , ( ). , , ( ), , , . ( denisgrim :" — , . — "). , . - (Dermabond, FDA 1998 , SurgiSeal). - Indermil, GluStitch, GluSeal, PeriAcryl, LiquiBand ( IDtmtm ). " Globus". .

, . , . , . , FDA- , (- ..).
. , .. ( ) (, , , ). , . . . 5% " " (= ), , .
""
, (, , , ) , . . , , (Permabond 910") . . ().

C —
spiritus_sancti : . , — , ( ). , , .

?
?
, . ( ) . , , , , . (, "" ), «» , . , γ- ( ).
/ Snarky
Glue is half the battle, the most interesting thing is how to unstick it :)
— — . — 3-5 4 . , , , . :)
1. . , , , ( ). .
2. . , . , , .… 100 . . . - , — 15-20 145
3. . , . , , ( 1/10 ) … . 12-24 . , . .
1: — / . , . Attention! — .
2: ( mphys )

NB " " — . —

Dean J (1985), Lange's Handbook of Chemistry, McGraw Hill, New York.
Drain KF, Guthrie J, Hung C, Martin F and Otterburn M (1984), 'Effect of moisture on the strength of steel-steel cyanoacrylate adhesive bonds', J. Adhesion, 17(1), 71-81
Grant E (1983), Drop by Drop: The Loctite Story, Loctite Corporation, Newington, CT.
Klemarczyk P and Okamoto Y (1993), 'Primers for bonding polyolefin substrates with alkyl cyanoacrylate adhesive', J. Adhesion, 40, 81-91
Lee H (1986), Cyanoacrylate Resins — The Instant Adhesives, Pasadena Technology Press, Los Angeles.
Rich R (1994), 'Anaerobic adhesives', in Handbook of Adhesive Technology, Pizzi A and Mittal K (eds), Marcel Dekker, New York, 467-79.
')
Source: https://habr.com/ru/post/452394/
All Articles
