About the caustic and not very
- These idiots put a porcelain container with “jelly” in a special camera, extremely isolated ... That is, they thought that the camera was extremely isolated, but when they opened the container with manipulators, the “jelly” went through the metal and plastic, like water through a blotter, broke loose outward, and everything with which it came into contact, again turned into a “jelly”. Thirty-five people were killed, more than a hundred were mutilated, and the entire laboratory building was rendered unusable. Have you ever been there? Great construction! And now the "jelly" stack in the basements and lower floors ... Here you have a prelude to the contact.
- A. Strugatsky, B. Strugatsky "Roadside Picnic"
Hi% username%!
That I'm still writing something - blame this person here . He inspired the idea.
')
Just a little thought, I decided that a little excursion into caustic substances will turn out relatively quickly. Maybe someone will be interested. And for someone it is useful.
Go.
Immediately determine the concepts.
Caustic - 1. Chewy. 2. Sharp, irritant, pain. 3. Stinging, caustic.
Ozhegov S.I. Dictionary of the Russian language. - M .: Rus.yaz., 1990. - 921 p.
So, we immediately discard the last two meanings of the word. We also discard the "caustic" lacrimators - which are not so much caustic as they cause lacrimation, and sternites - which cause coughing. Yes, below there will be substances that possess these properties, but they are what matters most! - really eat away materials, and sometimes flesh.
We will not consider substances that are corrosive only to humans and the like in view of the specific destruction of cell membranes. And therefore yprity remain out of work.
We will consider compounds that in room conditions are liquids. Therefore, liquid oxygen and nitrogen, as well as gases like fluorine will not be considered, although they can be considered caustic, yes.
As usual, the view will be exclusively subjective, based on personal experience. And yes - it is quite possible that I don’t remember anyone - write comments,% username%, within three days from the moment of publication I will supplement the article with what was forgotten from the very beginning!
And yes - I do not have the time and strength to build a “hit parade”, so there will be a hodgepodge. And with all the exceptions - it came out pretty short.
Caustic alkali
Specifically - alkali metal hydroxides: lithium, sodium, potassium, rubidium, cesium, France, thallium (I) hydroxide and barium hydroxide. But:
- Lithium, cesium, rubidium and barium are discarded - expensive and rarely seen
- If you,% username%, meet hydroxide France, then caustic will worry you last - it is terribly radioactive
- The same with thallium - it is poisonous to horror.
That is why sodium and potassium remained. But let's be frank - the properties of all caustic alkalis are very similar.
Sodium hydroxide is known to all as "caustic soda" (not to be confused with food, soda and other soda, as well as potash). Potassium hydroxide as a food additive E525 - too. The properties are both similar: highly hygroscopic, that is, they pull water, they "blur" in air. Well dissolved in water, with a large amount of heat.
"Spreading" in air is essentially the formation of very concentrated alkaline solutions. And therefore, if you put a piece of caustic alkali on paper, leather, some metals (the same aluminum), then as time passes it will be revealed that the material is well lifted! What was shown in “Fight Club” is very similar to the truth: indeed, sweaty hands - yes, in alkali - it will hurt! Personally, it seemed to me more painful than from hydrochloric acid (see below).
However, if the hands are very dry - most likely you will not feel anything exactly in the dry alkali.
Caustic alkalies perfectly break up fats into glycerin and salts of fatty acids - they make soap (hello, “Fight Club!”). Slightly longer, but proteins are also effectively broken down - that is, alkalis dissolve the alkali in principle, especially strong solutions - even when heated . The disadvantage in comparison with the same perchloric acid (about it, too, below) is that all alkalis pull carbon dioxide from the atmosphere, and therefore the force will gradually decrease. In addition, the alkali react with the components of the glass - the glass becomes cloudy, although, to dissolve it completely - here, of course, we must try.
Tetraalkylammonium hydroxides are sometimes referred to caustic alkalis, for example
Tetramethylammonium hydroxide

In fact, these substances combined the properties of cationic surfactants (well, it is just like ordinary soap - only cationic: here the active diphilic particle has a “+” charge, and in a soap it has a “-” charge) and a relatively high basicity. If it gets onto your hands, you can soap it in water and wash it like soap, if you warm your hair, skin or nails in an aqueous solution, it will dissolve. “Food” on the background of sodium and potassium hydroxides is so-so.
Sulphuric acid
H 2 SO 4
Probably the most popular in all stories. Not the most caustic, but rather unpleasant: concentrated sulfuric acid (which is 98%) is an oily liquid that loves water very much, and therefore takes it away from everyone. Taking water away from cellulose and sugar, charring them. In the same way, she will happily take water away from you,%% username, especially if you pour it on the delicate skin of the face or in the eyes (well, in fact, everything will come to your eyes with adventures). Particularly good people interfere with sulfuric acid and oil, so that it is harder to wash off and better absorbed into the skin.
By the way, taking away the water, sulfuric acid heats up great, which makes the picture even more juicy. So wash it off with water is a very bad idea. Better - oil (rinse, not rub - and then rinse with water). Well, or a large flow of water to immediately and cool.
“First, water, and then acid - otherwise a big trouble will happen!” - this is about sulfuric acid, although for some reason everyone thinks about any acid.
Being an oxidizing agent, sulfuric acid oxidizes the surface of metals to oxides. And since the interaction of oxides with acids takes place with the participation of water as a catalyst — and sulfuric acid does not give water — an effect called passivation occurs: a dense, insoluble and impermeable film of metal oxide protects it from further dissolution.
According to this mechanism, concentrated sulfuric acid is sent to distant distances of iron, aluminum. It is noteworthy that if the acid is diluted, water appears, and it cannot be sent, the metals dissolve.
By the way, sulfur oxide SO 3 is dissolved in sulfuric acid and it turns out oleum - which is sometimes mistakenly written as H 2 S 2 O 7 , but this is not entirely true. Oleum thirst for water is even greater.
Own sensations from getting sulfuric acid on the hand: a little warm, then a little bake - washed away under the tap, that's okay. Do not believe the films, but I do not advise you to drip on your face.
Organics often use chrompeak or “chrome mixture” - this is potassium dichromate dissolved in sulfuric acid. In fact, it is a solution of chromic acid, it is good for washing dishes from organic residues. When it comes to hand, it burns too, but in fact it is sulfuric acid plus toxic hexavalent chromium. Holes in the hand can not wait, except on the clothes.
The author of these lines is familiar with the idiot who used potassium permanganate instead of potassium dichromate. In contact with organic matter a little bit zhahnulo. Those present
By the way, since I remembered the chrome - we digress a bit from the topic of acids and
Chromyl chloride
CrO 2 Cl 2
In essence, it is a fierce compound of hexavalent chromium and hydrochloric acid. The dark red liquid that pulls water is hydrolyzed - and as a result it smokes with this very hydrochloric acid. The causticity is the result of this fraternal unity: chromium oxidizes, hydrochloric acid dissolves: it ignites some organic solvents (alcohol, turpentine), but in some it dissolves (carbon tetrachloride, dichloromethane, carbon disulfide). Eats up metals, but not as good as acids - again, the matter of passivation. for example, when exposed, steel acquires a beautiful dark blue surface.
It is clear to the skin, it ulcers, and this is stronger than chromic, since it penetrates the skin better than non-polar organic tissue. But this is not the case, but in hexavalent chromium, which is actually a carcinogen, and therefore penetrates deeper - more problems. And of course, breathing is much more dangerous.
Hydrochloric acid
HCl
Above 38% in water does not happen. One of the most popular acids for dissolution is that it is cooler than the others, because it can be very clean technologically, and besides acting as an acid, it also forms complex chlorides, which increase solubility. By the way, for this reason, insoluble silver chloride is very soluble in concentrated hydrochloric acid.
This skin burns a bit harder when getting on the skin, subjectively — it also itches, and it stinks: if you work a lot with concentrated hydrochloric acid in a laboratory with a bad hood, your dentist will say “thank you” to you: you will treat it with fillings. By the way, chewing gum helps. But not much. Better - hood.
Since it is not oily and does not heat up much with water, causticity is only for metals, and then not for everyone. By the way, steel in concentrated hydrochloric acid is passivated and says “not-so!” To it. What is used during transportation.
Nitric acid
HNO 3
It is also very popular, for some reason they are also afraid of it - but in vain. Concentrated - this is up to 70% - it is the most popular, above it is “fuming”, most often no one needs it. There is still water - so that also explodes.
Being an oxidizing agent, it passivates many metals, which are covered with an insoluble film and say: “goodbye” is chromium, iron, aluminum, cobalt, nickel and others.
With the skin, it instantly reacts according to the principle of the xantoprotein reaction - there will be a yellow spot, which means that you,%% username, are still made of protein! After some time, the yellow skin will peel off like a burn. At the same time, it burns less than salt, although it stinks no worse - and this time it is more toxic: the flying oxides of nitrogen are not very good for the body.
In chemistry, the so-called "nitrous mixture" is used - the most popular consists of sulfuric and nitric acids. Used in the synthesis, in particular in obtaining fun substance - pyroxylin. For causticity - the same brompik plus beautiful yellow skin.
There is also “royal vodka” - this is part of nitric acid into three parts of hydrochloric. Used to dissolve some metals, mostly precious. Drip method of checking samples of gold products is based on different ratios and adding water - by the way, it is very difficult to fool specialists with this method with fake. In terms of causticity for the skin, the same “nitrating mixture” plus stinks superbly, you can't confuse the smell with anything, it is also quite toxic.
There is also “reverse aqua regia” - when the ratio is the opposite, but this is a rare specificity.
By the way, about the very “fuming” that is red, angry and oxidizing - I quote the story of a good friend who sent me right now.
I drove this very 98% nitrogen. Either I simply overtook for cleaning, or from melange, I don’t remember. Capture two liters, rented receiver. I ask the technician to give a clean flask of 2 liters - pour. She gave me a dry, clean, but from under the alcohol - and with a closed stopper. That is, the pairs were accumulated. I go there funnel and pour. I have her there - and she is back. Well sprinkled on his hands, on the face and below the neck. Feeling - like an eagle in the face clutched. Plus hands, neck, under the nose, well, etc. on the little things. In my hands, I recall, two liters of the same kind. Eyes closed naturally. I understand that it is impossible to throw a flask; it will immediately be much worse. Carefully put the flask on the rubber stand, move to the sink, turn the gander into my face and turn on the full pressure. Seconds for five managed. To subcutaneous tissue is not reached. And then everything would be much worse. I saw another guy that happens in 10-15 seconds. Hard healing crimson scars on half hands. Then I understood why she was so angry. Not only is it quite a strong acid and oxidant, it is also a wonderful solvent. Unlimited mixed with water, but unlimited mixed with, for example, dichloroethane. Such a bifilnaya rubbish.
Phosphoric acid
H 3 PO 4
In fact, I gave the formula orthophosphoric acid - the most common. And there is still metaphosphoric, polyphosphoric, ultraphosphoric - in short, enough, but it does not matter.
Concentrated orthophosphoric acid (85%) is such a syrup. The acid itself is average, it is often used in the food industry, by the way - when they give you fillings, the surface of the tooth is first etched with phosphoric acid.
Corrosion is her so-so, but there is an unpleasant nuance: this syrup is well absorbed. Therefore, if it drips onto things, it will be absorbed, and then it will slowly erode. And if there is a stain or a hole from nitric acid and hydrochloric acid, then the thing will crack from the phosphoric acid, especially it is colorful on the shoes, when the hole seems to crumble until it turns through.
Well, in general, it is difficult to call it caustic.
Hydrofluoric acid
HF
Concentrated hydrofluoric acid is about 38%, although there are strange exceptions.
A weak acid that takes fluoride-furious love to form stable complexes with everything you can. Therefore, surprisingly dissolves the fact that other, stronger friends - can not, and therefore very often used in different mixtures for dissolution. If you touch the hand, you will feel more from other components of such mixtures, but there is a nuance.
Hydrofluoric acid dissolves SiO 2 . That is sand. That is, glass. That is quartz. Well, and so on. No, if you splash on the window with this acid - it will not dissolve, but a muddy stain will remain. To dissolve - you need to keep for a long time, and even better - to heat. When dissolved, SiF 4 is released , which is so good for health that it is better to do it under a hood.
A small but pleasant nuance: you have silicon,% username%, in your nails. So, if hydrofluoric acid gets under the nails - you will not notice anything. But at night you can not sleep - it will hurt like that, that sometimes there is a desire to tear off a finger. Believe me, friend - I know.
In general, hydrofluoric acid is toxic, carcinogenic, absorbed through the skin and a lot of everything - but we are today about causticity, right?
Remember, we agreed at the very beginning that fluorine would not be? It will not be. But there will be ...
Inert Gas Fluorides
In fact, fluorine is a harsh guy, you don’t particularly hang out with him, and therefore some inert gases form fluorides with him. Such stable fluorides are known: KrF 2 , XeF 2 , XeF 4 , XeF 6 . All of these are crystals that, in air at different speeds and hunts, are decomposed by moisture to hydrofluoric acid. Caustic - appropriate.
Hydroiodic acid
HI
The strongest (according to the degree of dissociation in water) binary acid. A strong reducing agent than organic chemists use. It oxidizes on air and becomes brown, which makes it dirty on contact. The sensation of contact - as from salt. Everything.
Perchloric acid
HClO 4
One of the strongest (in terms of the degree of dissociation in water) acids in general (superacids compete with it — about them below) —Gammet’s acidity function (numerical expression of the medium’s ability to donate protons to an arbitrary base, the smaller the stronger the acid) is - 13. Anhydrous is a strong oxidant, likes to explode, and is generally unstable. Concentrated (70% -72%) - the oxidizer is not worse, often used in the decomposition of biological objects. Decomposition is interesting and exciting because it can explode in the process: you need to make sure that there are no particles of coal, so that it does not boil too vigorously, etc. Perchloric acid is also rather dirty - it cannot be cleaned by sub-distillation, the infection explodes! Therefore, it is used infrequently.
In contact with the skin burns, sensations as if from salt. It stinks. When you see in the films that someone threw the body into a container with perchloric acid - and it dissolved, then yes, this is possible - but long or warm. If it is warm, it can jerk (see above). So be critical of cinema (I think I saw it in Cloverfield, 10).
By the way, the causticity of chlorine oxide (VII) Cl 2 O 7 and chlorine oxide (VI) Cl 2 O 6 is the result of the fact that with water these oxides form perchloric acid.
Now imagine that we decided to combine strong acidity in one compound - and fluoride caustic: take a molecule of perchloric or sulfuric acid - and replace all hydroxyl groups with fluorine on it! The rubbish will turn out to be rare: it will interact with water and similar compounds - and a strong acid and a hydrofluoric acid will immediately be produced at the reaction site. BUT?
Sulfur, bromine and iodine fluorides
Remember, we agreed to consider only liquids? For this reason, ClF 3 chlorofluoride trifluoride , which boils at +12 ° C, did not get into our article, although all the horror stories that it is terribly toxic, inflame glass, a gas mask and, when pouring 900 kilograms, eats 30 cm of concrete and a meter of gravel - all this is true. But we agreed - fluid.
However, there is a yellow liquid - iodine pentafluoride IF 5 , a colorless liquid - bromine BrF 3 trifluoride , light yellow - bromine pentafluoride BrF 5 , which are not worse. BrF 5 , for example, also dissolves glass, metals and concrete.
Similarly, among all the sulfur fluorides, liquid is only disulfur decafluoride (sometimes also called sulfur pentafluoride) - a colorless liquid with the formula S 2 F 10 . But at ordinary temperatures this compound is stable enough, it does not decompose with water, and therefore it is not particularly caustic. True, 4 times more toxic than phosgene with a similar mechanism of action.
By the way, it is said that iodine pentafluoride was a “special gas” for filling the atmosphere in the rescue shuttle in the last frames of the 1979 Alien film.
I even found it, looked closer and realized that Ripley lived there in such harsh conditions that the alien beast was just a nude.

Superacids
The term “superacid” was introduced by James Conant in 1927 to classify stronger acids than ordinary mineral acids. In some sources, perchloric acid is referred to superacid, although this is not so - it is a common mineral.
A number of superacids are mineral, to which halogen has been hooked: halogen pulls electrons upon itself, all atoms are very angry, and everything gets as usual hydrogen: it falls off in the form of H + - women: that's what the acid has become stronger.
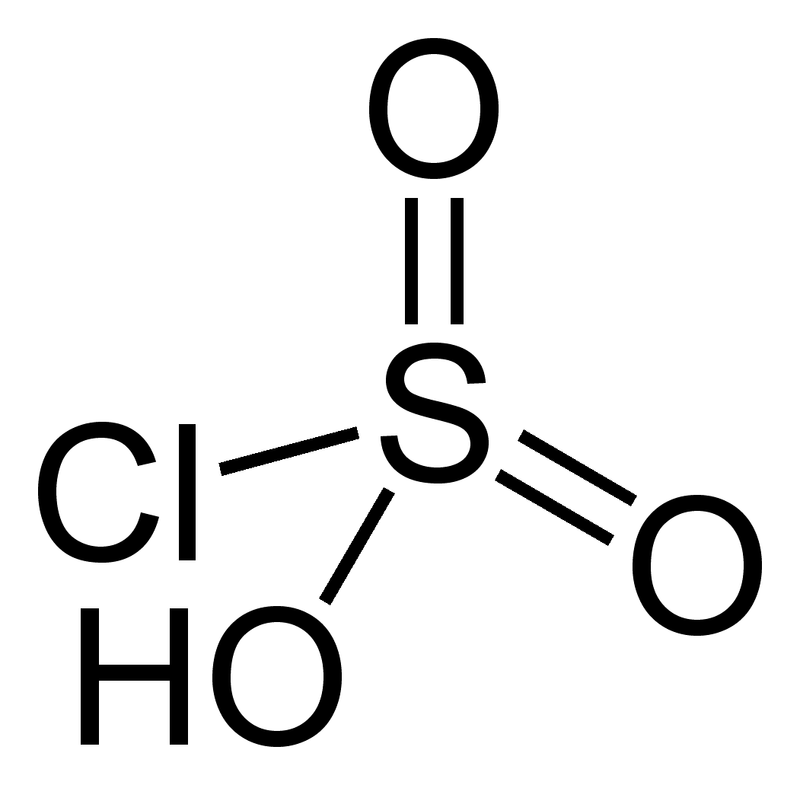
Examples are fluorosulfuric and chlorosulfuric acids.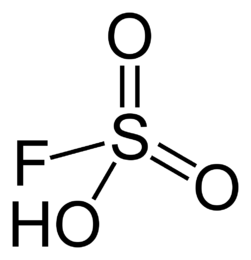


In fluorocarbonate, the Hammett function is -15.1, by the way, thanks to fluorine, this acid gradually dissolves the test tube in which it is stored.
Then one of the smart ones thought: let's take a Lewis acid (a substance that can accept a pair of electrons of another substance) and mix it with a Bronsted acid (a substance that a proton is capable of giving)! Antimony pentafluoride was mixed with hydrofluoric acid - received hexafluoroantimony acid HSbF 6 . In this system, hydrofluoric acid releases a proton (H + ), and the conjugate base (F - ) is isolated by coordination coordination with antimony pentafluoride. This forms a large octahedral anion (SbF 6 - ), which is a very weak nucleophile and a very weak base. Having become “free,” the proton causes the system to be extremely acidic — the Hammett -28 function!
And then others came and said, but why did they take the weak acid of Bernsted - and they came up with this.
Trifluoromethanesulfonic acid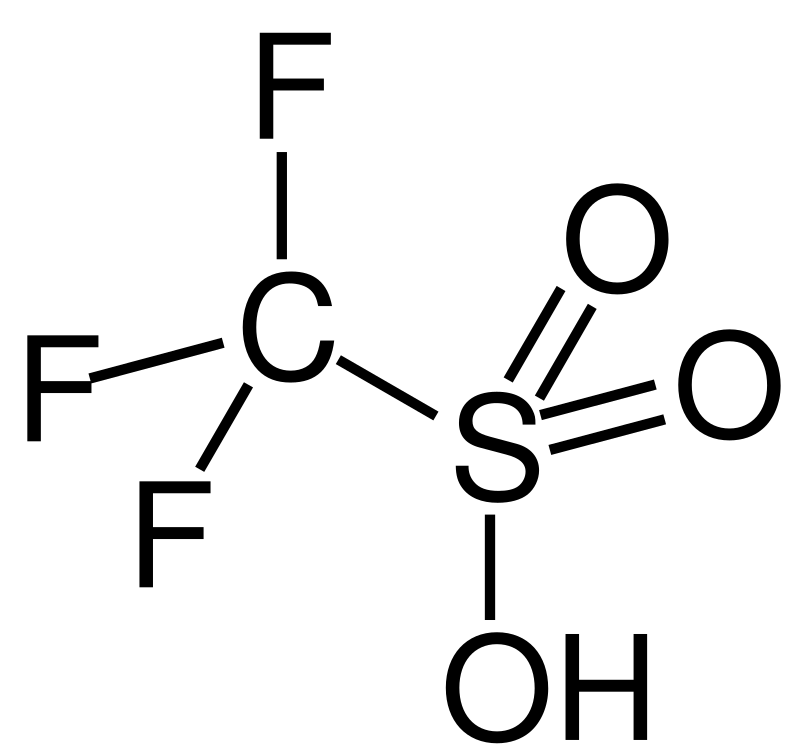

- in itself is already superacid (Hammett function -14.1). So, antimony pentafluoride was added to it again - they got a reduction to -16.8! The same focus with fluorocarbonate gave a reduction to -23.
And then a group of scientists from the Department of Chemical at the American University of California, led by Professor Christopher Reed, and colleagues from the Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences (Novosibirsk) came up with carboranic acid H (CHB 11 Cl 11 ). Well, "carboranic" it was called for ordinary people, and if you want to feel like a scientist - say "2,3,4,5,6,7,8,9,10,11,12-undeclor-1-carba-closo-dodecaboran (12) "three times and quickly.
It looks like this beauty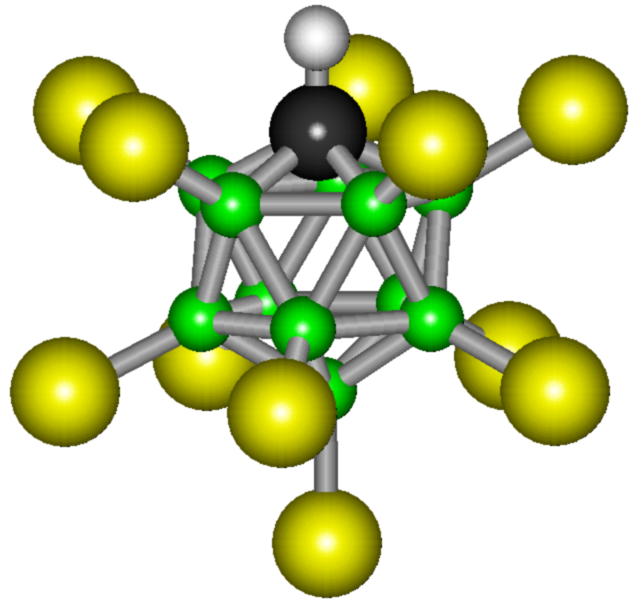

It is a dry powder that is soluble in water. This is the strongest acid at the moment. Carboranic acid is about a million times stronger than concentrated sulfuric acid. In normal scales, it is not possible to measure the strength of an acid, since acid protonates all known weak bases and all solvents in which it dissolves, including water, benzene, fullerene-60, sulfur dioxide.
Subsequently, Christopher Reed, in an interview with Nature News, said: “The idea of synthesizing carboranic acid was born from the fantasy“ of molecules never before created ”. Together with his colleagues, he wants to use carboranic acid to oxidize xenon inert gas atoms - simply because no one has done this before. Original what to say.
Well, since superacids are common acids, they usually act, only slightly stronger. It is clear that the skin will burn, but this does not mean - what to dissolve. Fluorosulfonic - a separate case, but there everything is due to fluorine, as in hydrofluoric.
Trihaloacetic acid
Specifically - trifluoroacetic and trichloroacetic acid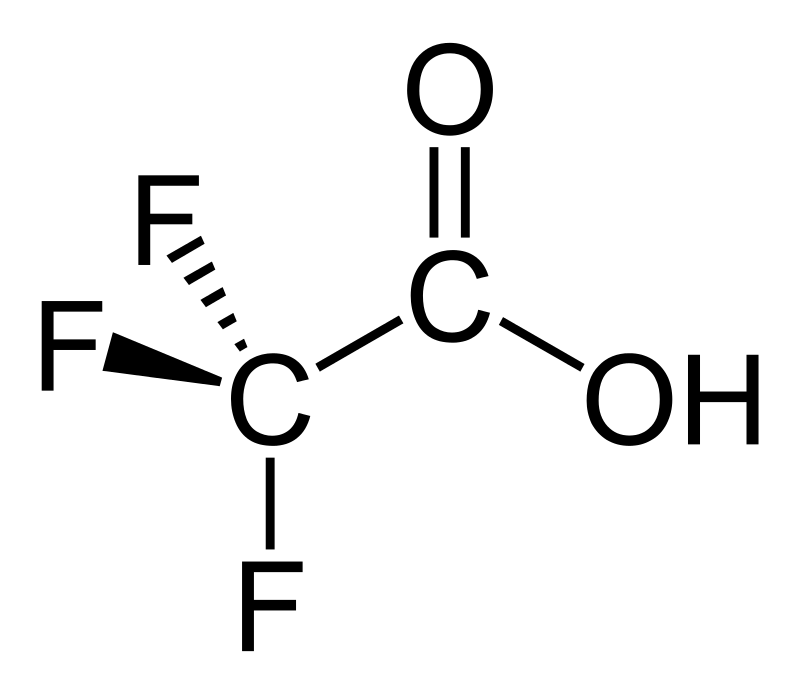
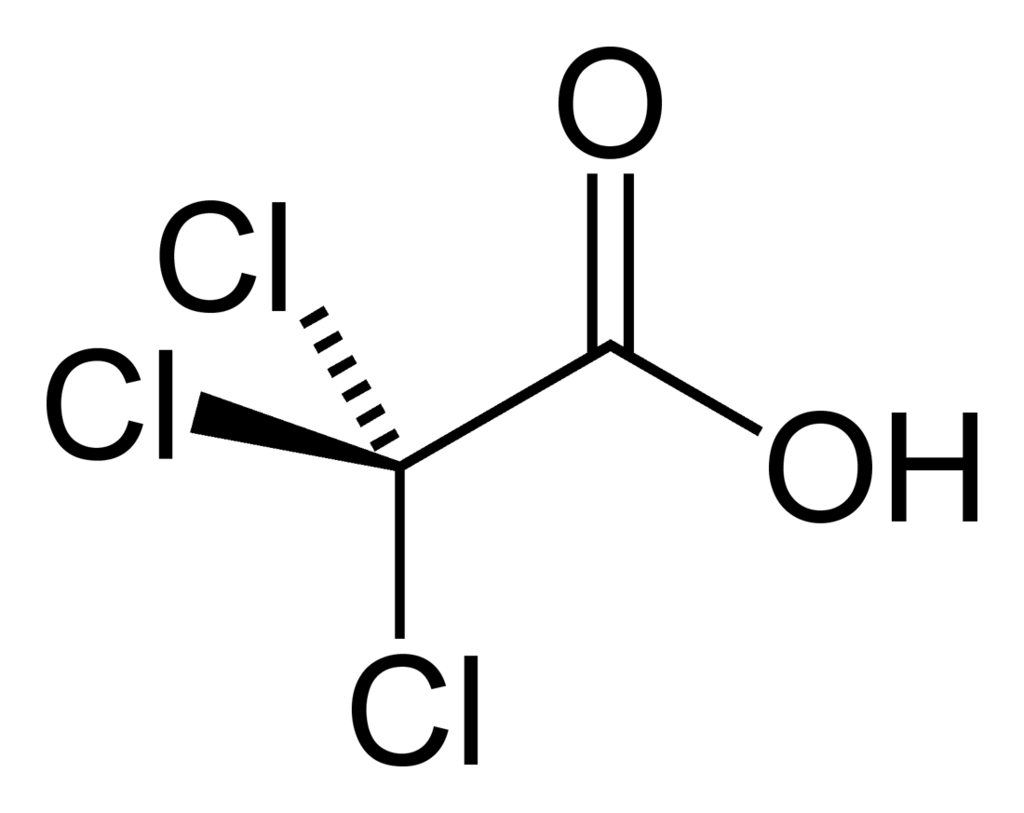


Lovely and pleasant combination of the properties of an organic polar solvent and a fairly strong acid. Stink - like vinegar.
The best thing is trifluoroacetic acid: a 20% solution destroys metals, cork, rubber, bakelite, polyethylene. On the skin burns and forms dry ulcers, reaching the muscle layer.
Trichloroacetic in this regard - the younger brother, but also nothing. By the way, the applause of the weaker sex: in pursuit of beauty, some go for the so-called TCA-peeling procedure (TCA is TetraChloroAcetate) - when the top coarsened skin is dissolved with this very trichloroacetic acid.
However, if the beautician swallows on the phone, fail is possible.

Acetic acid
CH 3 COOH
Most likely, you have this acid in your kitchen - and yes, it is used as a food additive E260. But it can also be stronger - a 70-80% aqueous solution of acetic acid is called acetic essence, and if the concentration is close to 100%, glacial acetic acid (because it can freeze and form something similar to ice.
Acetic acid is not so caustic with respect to metals as mineral acids, but since it is not so polar, and to some extent even diphilic (the combination of hydrophobic and hydrophilic parts in one molecule - as in surfactants), it is great absorbed by the skin. Solutions with a concentration of acetic acid greater than 30% are considered dangerous. The peculiarity of burns is that the development of coagulation necrosis of adjacent tissues of various lengths and depths is also initiated - if not washed away, then there will be a long healing ulcers and scars.
Well, it stinks, of course, notably.
Formic acid
NSAA
We have already discussed that formic acid, which is formed in the body after taking methanol, is one of the main causes of its toxicity. So, formic acid from the outside is not at all so dangerous, because it is rapidly metabolized and excreted by the body. Toxicity is quite low - for rats LD 50 about 1.8 g / kg, and therefore formic acid is also often used, including as a food additive - and this should not be feared.
"The acidity of formic acid depends on the concentration. According to the classification of the European Union, a concentration of up to 10% has an irritant effect, more than 10% is corrosive. And again, it’s not about metals and glass but about the body. When in contact with skin, 100% liquid formic acid causes severe chemical burns, hitting even a small amount on the skin causes severe pain, the affected area at first turns white, as if covered with frost, then becomes like wax, a red border appears around it. The fatty layer of the skin is cut, so washing the affected area with soda solution should be done immediately, so the ants really know something.
Bromine
Br 2
A heavy caustic liquid of red-brown color with a strong unpleasant odor, remotely resembling the smell of iodine and chlorine. By the way, the name "bromine" from the Greek βρῶμος - "stinker", "stinky."
Bromine is a typical halogen, according to its chemical activity, bromine is intermediate between chlorine and iodine. That is, not as quick as fluoride - but more alive than boring iodine. And yes, it does not reach chlorine either.
Slightly soluble in water, good - in some organic solvents. Bromine water - a reagent for unsaturated hydrocarbons - stinks, but quite peaceful and does not dissolve anything.
Pure bromine is
Concrete and glass to bromine are fairly stable. Organic compounds bromine - what? - right! - Brominated in the presence of an unsaturated bond. For this reason, the stability of polymers depends on their type, for example, polyethylene and polypropylene - they wanted to spit on bromine under room conditions.
Hydrogen peroxide
H 2 O 2
Unstable compound that is constantly falling apart into oxygen and water. The higher the concentration - the more unstable that gradually turns into an explosion hazard. To stabilize technical hydrogen peroxide, pyrophosphate or sodium stannate is added to it; when stored in aluminum containers use a corrosion inhibitor - ammonium nitrate.
Hydrogen peroxide in the laboratory is usually a solution of 38%. After contact with skin, it produces a chemical burn with characteristic white staining. The burn is painful, especially on thin skin, the whitened, horny skin then often cracks and itches.
In medicine, 3% hydrogen peroxide is used to clean deep wounds of a complex profile, purulent streaks, phlegmon and other purulent wounds, which are difficult to rehabilitate - so the substance has not only an antiseptic effect, but also creates a large amount of foam when interacting with the enzyme catalase. This in turn makes it possible to soften and separate necrotic areas, blood clots, pus from tissues, which will be easily washed away by the subsequent introduction of an antiseptic solution into the wound cavity. By the way, hydrogen peroxide is undesirable in other cases of wounds: having good cleansing properties, this substance does not really accelerate the healing process, because it damages the cells adjacent to the wound, as well as young, newly formed tissues - and this is also fraught with scarring.
Except for burns on the skin - nothing corrodes and does not dissolve. Metals, glass and plastics are resistant to hydrogen peroxide.
And hydrogen peroxide gave the world a lot of unique natural blondes with black hair roots!
The so-called peracids, acids in which peroxide groups are present, are close to hydrogen peroxide. Example: peracetic acid CH 3 UNSD is a substance resembling hydrogen peroxide in its properties, and therefore it is used in exactly the same areas. There is a “pervom” or “C-4” (no, this is not the C-4 you thought about) - it is HCOOOH permuramic acid , which is even weaker than peracetic acid , and therefore the Hirigs wash her hands before the operation. And finally - trifluoroperoacetic acid CF 3UNSD is a fierce, furious oxidizing agent that organic chemists look at with admiration for the possibility of oxidizing aniline to nitrobenzene, for obtaining hypervalent iodine in organic compounds, the Bayer-Williger reaction and other things that are not understandable to normal people. In terms of causticity, trifluoroacetic acid, mixed with hydrogen peroxide, is what actually is, and therefore presents a particular danger to hands, yes. Because of its high oxidative capacity, trifluoroperoacetic acid is not sold, but is usually obtained by admiring organic chemists, where necessary, by reacting trifluoroacetic anhydride with hydrogen peroxide.
Well, something like this, if we talk about fluid and causticity. Will there be any extras?
Source: https://habr.com/ru/post/451010/
All Articles