About yellow phosphorus and human panic nature

Hi,% username%.
As promised, here is an article-story about yellow phosphorus and how it gloriously burned under Lviv in Ukraine relatively recently.
Yes, I know - Google gives a lot of information about this accident. Unfortunately, most of what he gives out is not true, or, as eyewitnesses say, nonsense.
')
Let's figure it out!
Well, in the beginning - no one's favorite materiel, and she, by the way, is very important!
As boring Wikipedia tells us, phosphorus is one of the common elements of the earth's crust: its content is 0.08–0.09% of its mass. It is not found in the free state due to high chemical activity. Forms about 190 minerals, the most important of which are apatite Ca 5 (PO 4 ) 3 (F, Cl, OH), phosphite Ca 3 (PO 4 ) 2, and others. Phosphorus is part of the most important biological compounds - phospholipids. Contained in animal tissues, is part of proteins and other important organic compounds (ATP, DNA), is an element of life. Remember this,% username%, and we will go further.
Phosphorus in its pure form is white, red, black and metallic. This is called allotropic modifications - the weaker sex is very well versed in them, because a diamond can be distinguished from graphite by touch - and these are also allotropic modifications, only in carbon. In general, phosphorus is the same.
The hero of our story — yellow phosphorus — is in fact a crude white. Very often, "unrefined" - this means an admixture of red phosphorus, and not some creepy foreign elements.
Yellow phosphorus (as well as white) is the most real ad: highly poisonous (MAC in atmospheric air 0.0005 mg / m³), a flammable crystalline substance from light yellow to dark brown. Specific gravity is 1.83 g / cm³, melts at +43.1 ° C, boils at +280 ° C. It does not dissolve in water, oxidizes easily in air and self-ignites. It burns with a dazzling bright green flame with the release of thick white smoke - small particles of P4O10 tetraphosphorus decoxide. This is again a boring Wikipedia, but please,%% username%, remember this information too.
Now we will understand.
Well, first of all, despite the toxicity of phosphorus, it is extremely difficult to poison it for a very simple reason: it self-ignites in air. Very fast. And it burns, as has already been said, with a
Although you may object, you write: the lethal dose of yellow phosphorus for a person is 0.05-0.15 grams, it dissolves well in body fluids and is rapidly absorbed when ingested (by the way, red phosphorus is insoluble and therefore relatively unsaturated). Acute poisoning occurs when inhaling vapors of yellow phosphorus and / or when they enter the gastrointestinal tract. Poisoning is characterized by abdominal pain, vomiting, beautiful glowing in the dark vomit, smelling of garlic, diarrhea. Another symptom of acute yellow phosphorus poisoning is heart failure.
After reading this, for some reason I remembered about phosphine poisoning (the symptoms are very similar) and I thought hard - but not about the existence of yellow phosphorus vapors, but about the adequacy of an individual who saw a piece of unknown, smoking, glowing in the darkness - and immediately ate Well, that is.
By the way, to get a phosphorus solution in water of 3 mg / l - and this is a saturated solution, it no longer dissolves - it takes a week to shake a piece of phosphorus in water. Well, it was not I who invented it, so says GOST 32459-2013 - and this isn’t any Internet for you!
In general, in my opinion - the toxicity of phosphorus is greatly exaggerated. But he has other nuances. About them - below.
Phosphorus is burning, as the experts like working with it, according to the gimlet rule: that is, the burning piece eats into the surface on which it burns. In the table. In the metal. In the shoe. In hand. The reason is simple: the product of combustion - phosphorus oxide - is essentially an acid oxide, which immediately draws water, forming phosphoric acid. Phosphoric acid, though not as nyashnaya as sulfuric or hydrofluoric, but loves to eat no less - and therefore everything eats away. By the way, it is sometimes added to the toilet cleaner. A nice combination of high-temperature burning (up to 1300 ° C) and hot acid gives additional holes to your table, and if you’re not lucky, your body. And yes,% username% - it is very painful.
I have already stated many times and I will argue that there is no greater enemy to man than himself: of course, the properties of yellow phosphorus did not go unnoticed - and good people thought up to add it to incendiary ammunition, because it is very convenient when something suddenly lights up in air !
It looks very beautiful - you can admire









But people after such attacks do not look very nice - so it’s better not to look

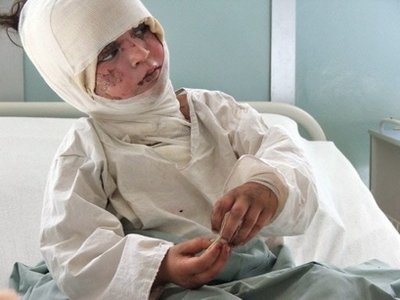
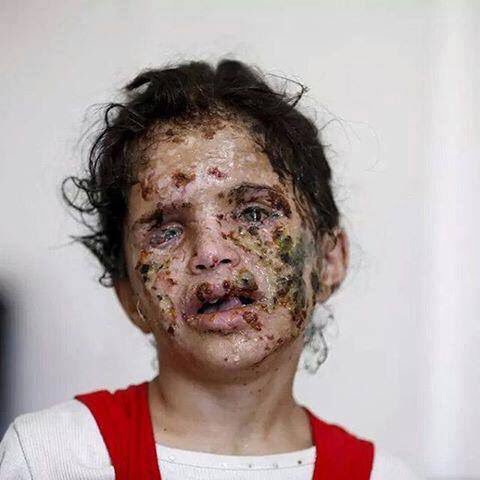
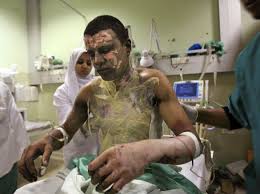





Since all this is very charming, the development, testing, transportation, trade, use and disposal of phosphorus ammunition are subject to a number of international agreements and treaties, including:
- St. Petersburg Declaration "On the abolition of the use of explosive and incendiary bullets" in 1868.
- The Additional Protocols of 1977 to the Geneva Convention for the Protection of Victims of the War of 1949, prohibiting the use of white phosphorus munitions if civilians are in danger as a result. The United States and Israel did not sign them, by the way.
- In accordance with the Third Protocol to the UN Convention on Specific Weapons of 1980, incendiary weapons should not be used against civilians, and, in addition, they cannot be used against military facilities that are in the zone of concentration of civilians.
In general, there are a lot of papers, but they have a status close to the toilet one, because pretty often these munitions are used - Palestine and Donbass will confirm.
Since phosphorus reacts with water only at temperatures above 500 degrees Celsius, water is used to extinguish phosphorus in large quantities (to reduce the temperature of the source of ignition and transfer phosphorus to a solid state) or copper sulfate solution (copper sulfate), after extinguishing the phosphorus fall asleep wet sand. To protect against spontaneous combustion, yellow phosphorus is stored and transported under a layer of water (calcium chloride solution, to be exact, but the water will also come down). This is also important!
Who produces phosphorus? And here,% username%, some will be imbued with pride: the main supplier of phosphorus, food phosphoric acid, hexaphosphate and sodium tripolyphosphate is proud Kazakhstan!
In fact, since the times of the USSR, the Kazphosphate enterprise was built in the glorious city of Dzhambul (yes, the name of Dzhambul Dzhabayev). Then Dzhambul was renamed Taraz - well, we will not discuss expediency, the Kazakhs know better, but the enterprise remains. The availability of raw materials and power, as well as the extremely low cost of labor (and more in Taraz / Dzhambul in fact, and nowhere to work) led to the fact that yellow phosphorus is done here.
When I was in this company, it was good there! South Kazakhstan, 300 km to Uzbekistan - warm! Birds are singing! Everything is green! On the horizon - the mountains! Beauty!
By the way, the Kazphosphate plant does not violate this idyll: it is all green, flowers, on the slope of a small mountain.
There really is good



The reason for beauty is simple - raw materials, product and waste products are phosphorus-containing substances, which are actually fertilizers. That's all and grows, blooms.
By the way, the highest bosses of the plant do not like dandelions. No one knows why. That is why before the visit of the highest authorities, workers organize a volunteer cleanup on dandelions. Well, as it is - to fight with dandelions - they all know about the country house / garden, within the framework of phosphorus lawlessness - it is completely meaningless: there is enough for a day, a maximum of two. But leadership is it.
I was particularly impressed by the work of the laboratory of the enterprise. There are really great clever people sitting there. And so that you understand% username%, a little bit of facts.
In yellow phosphorus it is very important to control impurities - especially arsenic, antimony, selenium, nickel, copper, zinc, aluminum, cadmium, chromium, mercury, lead, iron. To control all this - phosphorus needs to be dissolved, and at the same time everything that is controlled - should not fly away.
Task number one: how to weigh what lights up in the air? They do it like this: hammer a phosphorus ingot under a layer of water, take larger pieces — small ones flash too quickly — and transfer them to a glass of water. Then another glass is weighed with water, phosphorus is taken from the first one, wiped with alcohol, they wait until it dries - and they are thrown into a weighted glass with water. The weight difference determines the mass of phosphorus.
Since it can catch fire, there is a solution of copper sulphate next to it — if it caught fire, then throw it at it.
Then the phosphorus is dissolved. It dissolves in nitric acid, saturated with bromine vapor - a very sweet and aromatic thing, I recommend in the household (no). It is necessary to throw the phosphorus into this mixture, then heat it up a little, and when the reaction goes - transfer it to the trough with cold water, because the heating is enormous. And interfere-interfere-interfere - if not interfere, then the pieces just jump out of the boiling soup - the results will be inaccurate! They interfere with a hand, there are two mittens on it: rubber from acid - and felt from temperature (just rubber melts, but simply felt - does not save from acid drops. However, if phosphorus gets in, both of them will not save).
Fascinating spectacle of dissolving yellow phosphorus









Flying with the oxides of nitrogen and bromine - this is a note. The girls are afraid of these very red tails and pieces of phosphorus, which can get on the clothes or mitten. Poisoning by “vapor” or “solutions” of phosphorus is not remembered.
Yes, by the way, the salary of girls who do it is no more than $ 200 (and the solution is simple: there is no place to work in Taraz, I already said that). So the next time,% username%, when you whine about low wages and harmful work - remember Kazphosphate!
Well, now, when the basic knowledge has been accumulated, we now turn to the accident in Lviv.
Since phosphorus is in demand in Europe, Kazphosphate through Czech partners actively exports products. She rides in tanks filled with water, and it is clear that the rail.
On Monday, July 16, 2007, at 16:55 in the Busky district of the Lviv region of Ukraine on the Krasnoye-Azhidiv route, 15 tanks with yellow phosphorus of freight train No. 2005 turned over. In total, there were 58 cars. The tanks followed the Kazakh Asa station (Taraz, Kazakhstan) to the Okles station (Republic of Poland). Phosphorus leakage from one tank provoked spontaneous combustion of six other tanks.
It looked epic















And then - a mixture of panic, bloated media, lack of experience with yellow phosphorus and a complete ignorance of chemistry.
During extinguishing the fire, a cloud of burning products was formed with a lesion area of about 90 square kilometers. In this zone there are 14 settlements of the Busky district, where 11 thousand people live, as well as separate territories of the Radekhivsky and Brody districts of the region. The Ministry of Emergency Situations of Ukraine offered residents of nearby villages to evacuate and sent them about ten buses, but many people refused to leave their homes. Lviv authorities have assured that they will not forcibly evacuate anyone, although they warned about the unpredictability of the consequences of the accident. In total, about 800 residents are temporarily resettled out of the 6 settlements of the Busky District per night.
By Tuesday, there were 20 injured people (6 Emergencies Ministry specialists, 2 representatives of the Ministry of Internal Affairs, 2 railway workers and 10 people from the local population), 13 of them were hospitalized in a military and medical center of the Western operational command in Lviv in grave and moderately grave condition. Seven hospitalized - employees of the Ministry of Emergency Situations, two - employees of the State Automobile Inspectorate, four - local residents.
At the same time, the worst and wildest howl rose in the media. Some of the pearls are:
- My favorite, about coughing cats and dogs and the second Chernobyl
- Washington Post
- Vesti.RU
- BBC News
- Chron
- ... and over 9000, which Google finds with the right question
Reading all this - I'm sad. Because it shows an absolute ignorance of chemistry in the mass. And also - how easy it is to manipulate the uneducated mass (by the way,% username%, and you knew that slave owners in the United States firmly believed that slaves must be illiterate - so that they could not forge holiday certificates, other papers, correspondence with other settlements, coordinate uprisings etc. - little has changed).
More or less objective events in chronology are shown here (carefully - Ukrainian, if you do not know the shame - Google Translate):
What can be understood from this chronology?
- No one knew anything.
- Everyone wanted to popiaritsya.
- Fire / Emergencies were scared.
- The military too.
- Among the locals was a mess.
- Until representatives of Kazphosphate arrived on July 18, nobody understood what to do.
- No one wanted to pay for anything.
After talking with some employees of Kazphosphate who are directly involved in the aftermath of the accident, I can say the following.
There was no explosion / spontaneous combustion / phosphorus detonation - he quietly rode under water. And by itself, yellow phosphorus is not able to explode! But there was damage to the railroad tracks, because of which the tank got off the rail. When the tanks hit, a crack was formed, water flowed through it - well, and the phosphorus caught fire safely.
Temperature and features of burning finally destroyed the tank.
- White smoke is quite understandable - it is a pair of phosphoric acid, but not phosphorus. If you breathe them - yes, a strong cough will begin and in general it is not particularly useful. However, and not fatally harmful. Most of the injuries of the local population are due to the fact that people ran to collect interesting smoking pieces in water bottles, and they didn’t immediately put up a cordon - everyone was afraid.
- The fear of firefighters that supposedly “this rubbish from water burns!” Is due to the fact that a powerful jet of water smashed phosphorus into smaller pieces - well, they flew away and caught fire. It was necessary or a weak stream, or foam, which subsequently did.
- By the way, when everything was extinguished and only pieces were left inside the tank - the Kazakhs extinguished it. Well, it was extinguished - collected and thrown into a bucket of water to a greater extent. One of them is the chief technologist of the plant, a chain smoker. So - he quenched and smoked. Even the pictures of “a mad Kazakh, who also smokes in a terrible chemical fire, even went away in some places!” So what?
- There was no environmental catastrophe and the “second Chernobyl” and there could not be - in fact, nature received a dose of phosphate fertilizers.
- The only person who behaved adequately, listened to the Kazakhs and did as it should - Vladimir Antonets, First Deputy Minister of Emergency Situations. Probably because the colonel-general with a bunch of awards.
After it became clear that there was no sensation: there was no terrorist act, the threat of an environmental catastrophe, too, no one died
- The poor condition of the tracks on this railway line.
- Violation of safety rules by employees of the locomotive brigade.
- Negligence (ignored instructions for the temperature regime of transportation of especially dangerous goods).
- Inadequate technical condition of tanks.
In fact, the most truthful of this is the first. The rest was added in order not to pay the Kazakhs for the loss of cargo. Well, sort of insurance compensated.
So everyone stayed with their own.
Moral% username%: learn chemistry . She is everywhere. It will help you to live, and survive, and understand something for yourself.
And finally ...
Not all chemical compounds are harmful. Without hydrogen and oxygen, for example, it would be impossible to get water, the main component of beer.- Dave Barry, never a chemist
Source: https://habr.com/ru/post/450848/
All Articles