Each poison has its own antidote. How to escape or at least try (upd: about antidotes for household poisoning)
All RCBZ fighters (radiation, chemical and biological protection) who do not shame the honor of their OZK are dedicated to ...
With interest in reading the articles of the gjf colleague about the most interesting , scariest and most naughty poisons, I feel nostalgic :). Because any correct chemist, starting his career, is primarily interested in either poisons, or explosives, or drugs. I have not met people who would have been brought to chemistry by something else, honestly. It is true over the years, if the hobby becomes a profession, all information is somehow rethought and ordered, completely different priorities arise. And now, reading these articles, I recalled my paper notepad, which included descriptions of unusual poisons. Everything flows, everything changes, now in my “working” notebook there are more often “antidotes”. For man invented thousands of ways to destroy life, and not a single one has been invented yet - to create it. The force is in balance, so if there are poisons on Habré, there should be antidotes. Well, I woke up in me permanently dormant sergeant of the Belarusian RCBZ. The article is short, almost without water, but it may turn out to be vital (= “backfill”, methanol FAQ)! For the antidote - go under cat.

RU Wikipedia teaches us that:
In principle, every adult person at least once in their life heard about the antidote. Someone, for example, from an article about the gastric stone bezoar, which for centuries has been used as a remedy for any poison (“remedy”). Someone will remember Rasputin, who ate sweet cakes and thus saved himself from cyanide poisoning (but this did not save him from a bullet ), and someone Nicolas Cage from the movie The Rock, driving an attorney autodirector into the heart.
')
We have to admit that today antidotes as such have lost their relevance and are most often associated with the courses (posters on the walls of old scientific research institutes ) of civil defense, or with films of appropriate (terrorist, etc.) subjects. This happened because intensive care in emergency medicine reached unprecedented heights. Any poisoning is usually evaluated and treated symptomatically, consistently blocking or removing the damaging factors. However, the use of antidotes gives an advantage in reducing the time of development of poisoning and reducing complications, increases the possibility of recovery of the poisoned patient and saves his vital resources. When the possibilities for full-fledged intensive care are not available, some antidotes may become essential medicines, especially in remote areas or developing regions.
Until a certain point, all antidotes were not classified and existed completely isolated. But the situation changed in 1993, when, within the framework of the International Program on Chemical Safety (IPCS) of WHO, a new definition of the antidote was given:
and secondly, a list was prepared and submitted to the public, Guidelines for Poison Control . I recommend it, just in case, download and keep it in a prominent place, even though the IPCS experts constantly conduct research and clarify the information (= add to the list, etc.).
The list consists of the following tables:
tab. 1) 48 antidotes that have a positive effect in the treatment of certain acute poisoning
tab. 2) 12 substances used to prevent the absorption of poisons. They also provide symptomatic treatment.
tab. 3) 19 therapeutic agents that have a positive effect in acute poisoning
tab. 4) 23 antidotes and related therapeutic substances that are outdated and whose use is not currently recommended due to inefficiency.
I will give in the article only the first and last tables, as the most vital. The rest, if desired, the inquisitive reader will be able to see for himself, following the link mentioned above.
It should be noted that the WHO definition of a concept is very broad. It
includes both antidotes themselves and non-specific drugs (for example, glucose, vitamin K, diazepam, isoprenaline, etc.), which are widely used in the treatment of specific poisonings.
In the table of antidotes in the column "code" the letter shows the urgency of use:
A - you need to use immediately (within the first 30 minutes from the moment of poisoning),
B - you need to use immediately (in the first 2 hours),
C - it is necessary to use immediately (in the first 6 hours).
The number next to the letter identifies such a parameter as the proof of the effectiveness of the drug: 1 - the effectiveness of the antidote is well documented, 2 - the antidote is widely used, however additional research is needed on the effectiveness and indications for use, 3 - effectiveness is questionable.
Important note: Specific antidotes should be used only when established poisoning with the corresponding specific poison, and it is logical that to effectively eliminate the negative effects of poisoning, antidotes should be applied as quickly as possible (as soon as possible).
Addition : in response to "from the whole table - I know only 10-15 pieces ...". A few thoughts. For example, the antidote for thallium is Prussian blue , or Prussian blue. Chemist to get the blue of azure is not difficult, the benefit of " yellow blood salt " and ferric salts are almost everywhere. Ferrotsin tablets can help the man in the street (initially positioned as a sorbent of radioactive cesium), which after the Chernobyl disaster could still be found, and then suddenly it became difficult. In the extreme case, you can try to look for a veterinary drug Bifezh, also intended for feeding animals in areas contaminated with radionuclides. As an emergency option - there is watercolor paint , where the azure blue is used as a blue pigment. Note that yellow blood salt is a food additive E536, which is put, for example, in sprats in Belarusian-made tomato :). Sodium thiosulfate - a photoreactive agent common in the past, so-called. fixative (simple) neutral. Edetates are salts of EDTA, metal complexes with Trilon-B (EDTA), which is used to soften water and is sold in every stall. One of the best complexing agents is an excellent chelator for many heavy metals. Methionine is a sulfur-containing amino acid found in many foods (see USDA ). Methylene blue is a common household blueprint, which is not a problem to get. Amylnitrite is poppers , which can also be found in a large city without any problems. Etc. etc., if you look at it - everything is not so sadly it turns out :). In the appendage, briefly on the treatment of available antidotes:
Methanol / ethylene glycol poisoning (antifreeze)
The specific antidote to methanol is ethyl alcohol. Given the slow metabolism of methanol, ethanol is taken within 5 days from the time of consumption of methanol. Dose of ethanol: 1 - 2 g / kg of body weight per day. The optimal concentration of ethanol in the blood is 1. Ethanol is injected either intravenously dropwise (in the absence of consciousness, or vomiting) in the form of a 5% solution (20 ml of a 96% solution per 400 ml of a 5% glucose solution) at a rate of 100-150 mg / kg / h or orally as 30% solution every 3 hours, while the daily dose is evenly distributed between doses. To speed up the metabolism of formic acid, folic acid is injected 50 to 100 mg 4-6 times a day. When ethylene glycol poisoning is the same. At the earliest opportunity - immediately to the hospital with a description of the manipulations.
UPDATE: After a small FAQ on the definition of methanol, to dot the i
Q : Is it possible to distinguish pure methanol from pure ethanol by smell?
A : Yes, but very difficult. The method does not work in the case of a mixture of methanol + ethanol
Q : Do chemical methods exist to distinguish methanol from ethanol?
A : Pure - yes, for example, iodoform sample: the formation of a yellowish precipitate of iodoform under the action of iodine and alkali on alcohol (sensitivity> = 0.05%).
C 2 H 5 OH + 6NaOH + 4I 2 => CHI 3 + HCOONa + 5NaI + H 2 O
Add the Lugol solution to the test alcohol, mix and add the alkali solution (NaOH) dropwise. In the case of ethanol, the solution first becomes discolored, and then becomes cloudy, a yellow suspension of iodoform is formed, at high concentrations of alcohol a yellow precipitate is formed. Methanol does not give such a reaction.
The second option may be the oxidation of alcohol with copper oxide. The copper wire rubbed to a shine is calcined in a burner flame until blackened, then dipped in the alcohol under study. In the case of methanol, the reaction proceeds:
CH 3 OH + CuO => H 2 C = O + Cu + H 2 O (formaldehyde is formed and the wire becomes shiny)
In the case of ethanol, the reaction proceeds:
2 H 5 OH + CuO => CH 3 -CH = O + Cu + H 2 O (acetaldehyde is formed and the wire becomes shiny)
The method is complicated by the fact that the tester must know how clean aldehydes smell (acetic - reminds someone the smell of rotten apples, someone has a strong smell of fumes, formaldehyde - irritates the nasal mucosa, a very strong smell that can be sensed, for example, when decomposing phenol formaldehyde resin ).
The sad thing is that the voiced methods are not applicable in the case of an ethanol-methanol mixture. The old laboratory method of determination is the oxidation reaction of a mixture of alcohols with potassium permanganate in the presence of phosphoric acid and the indication of the formaldehyde formed with chromotropic acid. The reaction proceeds:
5CH 3 OH + 3H 3 PO 4 + 2KMnO 4 => 5HCOH + 2MnHPO 4 + K 2 HPO 4 + 8H 2 O
Formaldehyde from methanol gives chromotropic acid a violet color. Acetaldehyde reaction does not interfere.
Old GOST 5964-93 recommends the following methodology:
There are no other “express” options (if pressed, it is better to carry it on the chromatograph). So if there is no desire to understand, it is better to pour or burn such a mixture. If the volumes of the mixture are serious, the mixture can be separated by distillation (temperatures are seriously different + methanol does not form azeotropes with water). The boiling point of methanol is 64.7 ° C and that of ethanol is 78.39 ° C (78.15 ° C for rectified alcohol containing at least 4.43% water).
In addition, having at hand a fairly accurate portable refractometer:

and knowing the exact concentration of alcohol, methanol from ethanol can be tried to distinguish by the refractive index, for methanol nD 20 1.3288, for ethanol nD 20 1.3611
Paracetamol poisoning
Application in the first 16 hours of acetylcysteine (mucomist, mucosolvin). The initial dose of acetylcysteine is 140 mg / kg orally, then at 70 mg / kg every 4 hours for 3 days (another 17 doses). The antidote can also be administered intravenously (in particular, with severe vomiting), but the oral route is more effective and is associated with fewer side effects.
Iron poisoning
The antidote is the chelating agent desferal, which is administered intravenously or
intramuscularly. In patients with initial manifestations of toxicity (50-60 mcg / l of plasma iron) - administration of 10-15 mg / kg / hour of desferal. High doses - 40-50 mg / kg / hour apply
only with severe poisoning. The administration of deferoxamine continues until the plasma level of iron decreases below 35 µg / L. Do not use in the case of pregnant women with acute overdose of iron supplements.
Iodine poisoning
Sodium thiosulfate 30% solution - up to 300 ml in a day intravenous drip, 10% solution of sodium chloride 30 ml intravenously.
Manganese poisoning (potassium permanganate)
With acute cyanosis (methemoglobinemia) - methylene blue 50 ml of 1% solution, ascorbic acid - 30 ml of 5% solution intravenously.
Copper poisoning
Unithiol - 10 ml of 5% solution, then 5 ml every 3 h intramuscularly for 3 - 5 days. Sodium thiosulfate - 100 ml of a 30% solution intravenously.
Lead poisoning
Thetacin-calcium (CaEDTA) at a dose of 50 mg / kg / day) divided into 3-4 doses intramuscularly. Unithiol in 5-10 ml of 5% solution intramuscularly 4 times a day for 5 days. Orally administered DMSA (succimer) at 10 mg / kg every 8 hours for 5 days or 12 hours for 14 days.
Poisoning poisonous mushrooms with hepatotropic poisons (amatoxins) - pale toadstool,
mucky fly agaric, smelly fly agaric
Penicillin 1 million U / kg / day. Silibinin (legalon) - 20 mg / kg / day. When using preparations containing silymarin (silibor, Kars), it should be remembered that 70 mg of silymarin approximately correspond in effectiveness to 30 mg of silibinin.
Disulfiram poisoning - a drug used in the treatment of alcoholism. Actual for "podshivshis"
Intravenous administration of 40% glucose solution - 40 ml with 5% ascorbic acid solution - 10 ml. Sodium bicarbonate - 4% solution 200 ml - intravenously. Vitamin B 1 - 5% solution - 2 ml intramuscularly.
Poisoning by nitrites / nitrates, benzene, aniline, nitrogen oxides and other methemoglobin formers.
1% solution of methylene blue 0.1-0.2 ml / kg (1-2 mg / kg) with 5% glucose solution 200-300 ml intravenously, if necessary, again in 15-20 minutes. Ascorbic acid solution 5% to 60 ml per day intravenously. In the case of benzene - 30% sodium thiosulfate solution - 200 ml intravenously.
PS If something is not found - we write in the comments, we will try to figure out and disperse habr by antidotology.
With interest in reading the articles of the gjf colleague about the most interesting , scariest and most naughty poisons, I feel nostalgic :). Because any correct chemist, starting his career, is primarily interested in either poisons, or explosives, or drugs. I have not met people who would have been brought to chemistry by something else, honestly. It is true over the years, if the hobby becomes a profession, all information is somehow rethought and ordered, completely different priorities arise. And now, reading these articles, I recalled my paper notepad, which included descriptions of unusual poisons. Everything flows, everything changes, now in my “working” notebook there are more often “antidotes”. For man invented thousands of ways to destroy life, and not a single one has been invented yet - to create it. The force is in balance, so if there are poisons on Habré, there should be antidotes. Well, I woke up in me permanently dormant sergeant of the Belarusian RCBZ. The article is short, almost without water, but it may turn out to be vital (= “backfill”, methanol FAQ)! For the antidote - go under cat.

RU Wikipedia teaches us that:
An antidote or antidote (from ancient Greek. Ἀντίδοτον, literally - given against) is a drug that stops or weakens the effect of the poison on the body.
In principle, every adult person at least once in their life heard about the antidote. Someone, for example, from an article about the gastric stone bezoar, which for centuries has been used as a remedy for any poison (“remedy”). Someone will remember Rasputin, who ate sweet cakes and thus saved himself from cyanide poisoning (
')
We have to admit that today antidotes as such have lost their relevance and are most often associated with the courses (
Until a certain point, all antidotes were not classified and existed completely isolated. But the situation changed in 1993, when, within the framework of the International Program on Chemical Safety (IPCS) of WHO, a new definition of the antidote was given:
Antidote is a therapeutic substance used to counteract
side effects of xenobiotics.
and secondly, a list was prepared and submitted to the public, Guidelines for Poison Control . I recommend it, just in case, download and keep it in a prominent place, even though the IPCS experts constantly conduct research and clarify the information (= add to the list, etc.).
Cover Guidelines for Poison Control

The list consists of the following tables:
tab. 1) 48 antidotes that have a positive effect in the treatment of certain acute poisoning
tab. 2) 12 substances used to prevent the absorption of poisons. They also provide symptomatic treatment.
tab. 3) 19 therapeutic agents that have a positive effect in acute poisoning
tab. 4) 23 antidotes and related therapeutic substances that are outdated and whose use is not currently recommended due to inefficiency.
I will give in the article only the first and last tables, as the most vital. The rest, if desired, the inquisitive reader will be able to see for himself, following the link mentioned above.
It should be noted that the WHO definition of a concept is very broad. It
includes both antidotes themselves and non-specific drugs (for example, glucose, vitamin K, diazepam, isoprenaline, etc.), which are widely used in the treatment of specific poisonings.
In the table of antidotes in the column "code" the letter shows the urgency of use:
A - you need to use immediately (within the first 30 minutes from the moment of poisoning),
B - you need to use immediately (in the first 2 hours),
C - it is necessary to use immediately (in the first 6 hours).
The number next to the letter identifies such a parameter as the proof of the effectiveness of the drug: 1 - the effectiveness of the antidote is well documented, 2 - the antidote is widely used, however additional research is needed on the effectiveness and indications for use, 3 - effectiveness is questionable.
Table Antidotes from IPCS
Legend:

TABLE ANTIDOTS
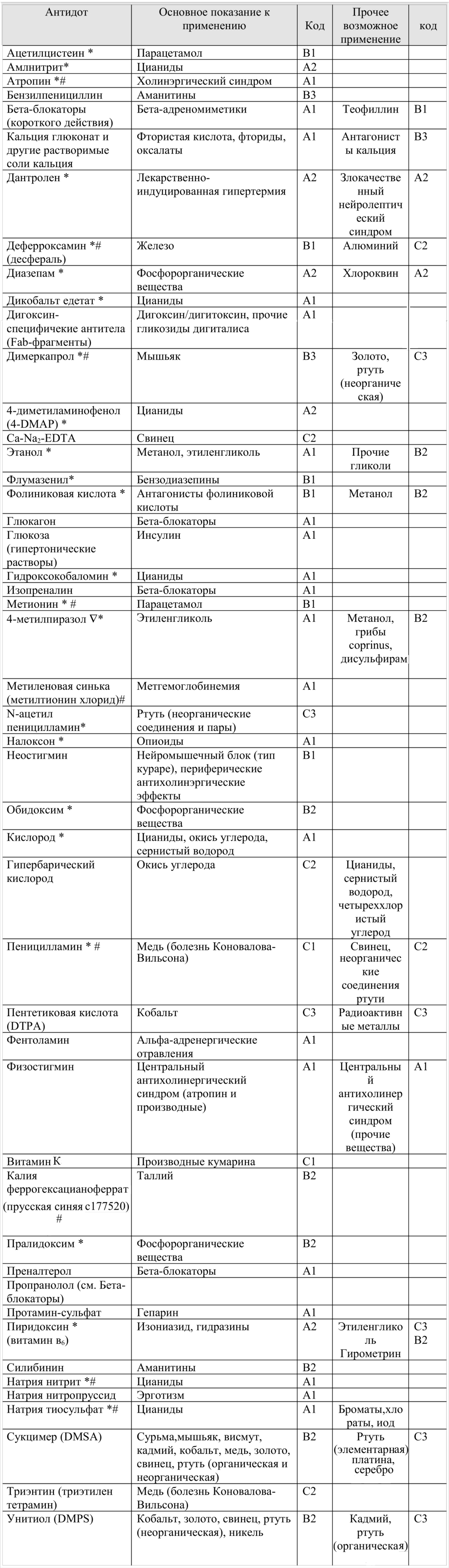

TABLE ANTIDOTS

Outdated, ineffective and dangerous antidotes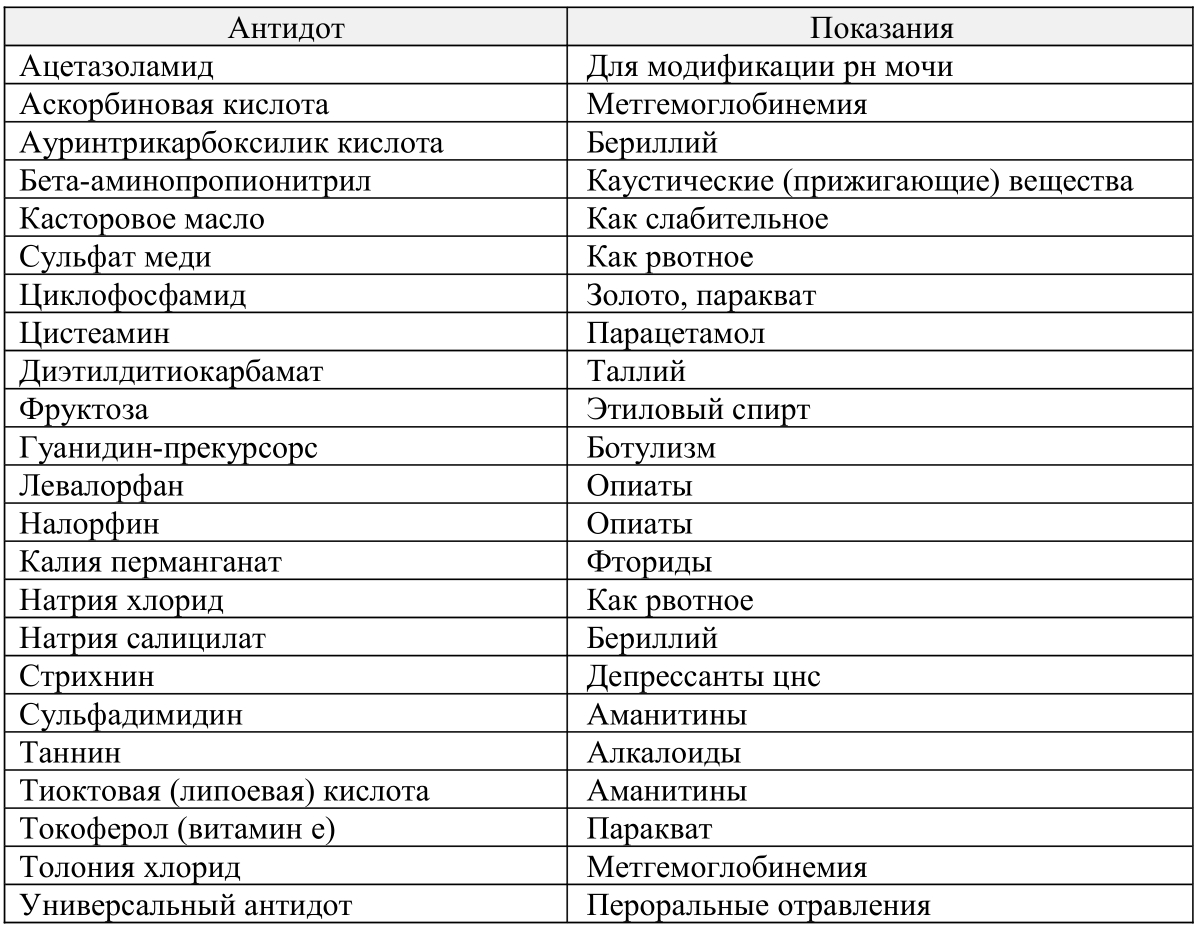

Important note: Specific antidotes should be used only when established poisoning with the corresponding specific poison, and it is logical that to effectively eliminate the negative effects of poisoning, antidotes should be applied as quickly as possible (as soon as possible).
Addition : in response to "from the whole table - I know only 10-15 pieces ...". A few thoughts. For example, the antidote for thallium is Prussian blue , or Prussian blue. Chemist to get the blue of azure is not difficult, the benefit of " yellow blood salt " and ferric salts are almost everywhere. Ferrotsin tablets can help the man in the street (initially positioned as a sorbent of radioactive cesium), which after the Chernobyl disaster could still be found, and then suddenly it became difficult. In the extreme case, you can try to look for a veterinary drug Bifezh, also intended for feeding animals in areas contaminated with radionuclides. As an emergency option - there is watercolor paint , where the azure blue is used as a blue pigment. Note that yellow blood salt is a food additive E536, which is put, for example, in sprats in Belarusian-made tomato :). Sodium thiosulfate - a photoreactive agent common in the past, so-called. fixative (simple) neutral. Edetates are salts of EDTA, metal complexes with Trilon-B (EDTA), which is used to soften water and is sold in every stall. One of the best complexing agents is an excellent chelator for many heavy metals. Methionine is a sulfur-containing amino acid found in many foods (see USDA ). Methylene blue is a common household blueprint, which is not a problem to get. Amylnitrite is poppers , which can also be found in a large city without any problems. Etc. etc., if you look at it - everything is not so sadly it turns out :). In the appendage, briefly on the treatment of available antidotes:
Methanol / ethylene glycol poisoning (antifreeze)
The specific antidote to methanol is ethyl alcohol. Given the slow metabolism of methanol, ethanol is taken within 5 days from the time of consumption of methanol. Dose of ethanol: 1 - 2 g / kg of body weight per day. The optimal concentration of ethanol in the blood is 1. Ethanol is injected either intravenously dropwise (in the absence of consciousness, or vomiting) in the form of a 5% solution (20 ml of a 96% solution per 400 ml of a 5% glucose solution) at a rate of 100-150 mg / kg / h or orally as 30% solution every 3 hours, while the daily dose is evenly distributed between doses. To speed up the metabolism of formic acid, folic acid is injected 50 to 100 mg 4-6 times a day. When ethylene glycol poisoning is the same. At the earliest opportunity - immediately to the hospital with a description of the manipulations.
UPDATE: After a small FAQ on the definition of methanol, to dot the i
Q : Is it possible to distinguish pure methanol from pure ethanol by smell?
A : Yes, but very difficult. The method does not work in the case of a mixture of methanol + ethanol
Q : Do chemical methods exist to distinguish methanol from ethanol?
A : Pure - yes, for example, iodoform sample: the formation of a yellowish precipitate of iodoform under the action of iodine and alkali on alcohol (sensitivity> = 0.05%).
C 2 H 5 OH + 6NaOH + 4I 2 => CHI 3 + HCOONa + 5NaI + H 2 O
Add the Lugol solution to the test alcohol, mix and add the alkali solution (NaOH) dropwise. In the case of ethanol, the solution first becomes discolored, and then becomes cloudy, a yellow suspension of iodoform is formed, at high concentrations of alcohol a yellow precipitate is formed. Methanol does not give such a reaction.
The second option may be the oxidation of alcohol with copper oxide. The copper wire rubbed to a shine is calcined in a burner flame until blackened, then dipped in the alcohol under study. In the case of methanol, the reaction proceeds:
CH 3 OH + CuO => H 2 C = O + Cu + H 2 O (formaldehyde is formed and the wire becomes shiny)
In the case of ethanol, the reaction proceeds:
2 H 5 OH + CuO => CH 3 -CH = O + Cu + H 2 O (acetaldehyde is formed and the wire becomes shiny)
The method is complicated by the fact that the tester must know how clean aldehydes smell (acetic - reminds someone the smell of rotten apples, someone has a strong smell of fumes, formaldehyde - irritates the nasal mucosa, a very strong smell that can be sensed, for example, when decomposing phenol formaldehyde resin ).
The sad thing is that the voiced methods are not applicable in the case of an ethanol-methanol mixture. The old laboratory method of determination is the oxidation reaction of a mixture of alcohols with potassium permanganate in the presence of phosphoric acid and the indication of the formaldehyde formed with chromotropic acid. The reaction proceeds:
5CH 3 OH + 3H 3 PO 4 + 2KMnO 4 => 5HCOH + 2MnHPO 4 + K 2 HPO 4 + 8H 2 O
Formaldehyde from methanol gives chromotropic acid a violet color. Acetaldehyde reaction does not interfere.
Old GOST 5964-93 recommends the following methodology:
Methodology GOST 5964-93

There are no other “express” options (if pressed, it is better to carry it on the chromatograph). So if there is no desire to understand, it is better to pour or burn such a mixture. If the volumes of the mixture are serious, the mixture can be separated by distillation (temperatures are seriously different + methanol does not form azeotropes with water). The boiling point of methanol is 64.7 ° C and that of ethanol is 78.39 ° C (78.15 ° C for rectified alcohol containing at least 4.43% water).
In addition, having at hand a fairly accurate portable refractometer:

and knowing the exact concentration of alcohol, methanol from ethanol can be tried to distinguish by the refractive index, for methanol nD 20 1.3288, for ethanol nD 20 1.3611
Paracetamol poisoning
Application in the first 16 hours of acetylcysteine (mucomist, mucosolvin). The initial dose of acetylcysteine is 140 mg / kg orally, then at 70 mg / kg every 4 hours for 3 days (another 17 doses). The antidote can also be administered intravenously (in particular, with severe vomiting), but the oral route is more effective and is associated with fewer side effects.
Iron poisoning
The antidote is the chelating agent desferal, which is administered intravenously or
intramuscularly. In patients with initial manifestations of toxicity (50-60 mcg / l of plasma iron) - administration of 10-15 mg / kg / hour of desferal. High doses - 40-50 mg / kg / hour apply
only with severe poisoning. The administration of deferoxamine continues until the plasma level of iron decreases below 35 µg / L. Do not use in the case of pregnant women with acute overdose of iron supplements.
Iodine poisoning
Sodium thiosulfate 30% solution - up to 300 ml in a day intravenous drip, 10% solution of sodium chloride 30 ml intravenously.
Manganese poisoning (potassium permanganate)
With acute cyanosis (methemoglobinemia) - methylene blue 50 ml of 1% solution, ascorbic acid - 30 ml of 5% solution intravenously.
Copper poisoning
Unithiol - 10 ml of 5% solution, then 5 ml every 3 h intramuscularly for 3 - 5 days. Sodium thiosulfate - 100 ml of a 30% solution intravenously.
Lead poisoning
Thetacin-calcium (CaEDTA) at a dose of 50 mg / kg / day) divided into 3-4 doses intramuscularly. Unithiol in 5-10 ml of 5% solution intramuscularly 4 times a day for 5 days. Orally administered DMSA (succimer) at 10 mg / kg every 8 hours for 5 days or 12 hours for 14 days.
Poisoning poisonous mushrooms with hepatotropic poisons (amatoxins) - pale toadstool,
mucky fly agaric, smelly fly agaric
Penicillin 1 million U / kg / day. Silibinin (legalon) - 20 mg / kg / day. When using preparations containing silymarin (silibor, Kars), it should be remembered that 70 mg of silymarin approximately correspond in effectiveness to 30 mg of silibinin.
Disulfiram poisoning - a drug used in the treatment of alcoholism. Actual for "podshivshis"
Intravenous administration of 40% glucose solution - 40 ml with 5% ascorbic acid solution - 10 ml. Sodium bicarbonate - 4% solution 200 ml - intravenously. Vitamin B 1 - 5% solution - 2 ml intramuscularly.
Poisoning by nitrites / nitrates, benzene, aniline, nitrogen oxides and other methemoglobin formers.
1% solution of methylene blue 0.1-0.2 ml / kg (1-2 mg / kg) with 5% glucose solution 200-300 ml intravenously, if necessary, again in 15-20 minutes. Ascorbic acid solution 5% to 60 ml per day intravenously. In the case of benzene - 30% sodium thiosulfate solution - 200 ml intravenously.
PS If something is not found - we write in the comments, we will try to figure out and disperse habr by antidotology.
Source: https://habr.com/ru/post/450568/
All Articles