HIV - treatment methods from first drugs to today
Before proceeding to the presentation of the material, I would like to say a few words about myself: a community participant in the fight against HIV denial („HIV / AIDS dissidence“): in 2016-2018 „HIV / AIDS dissidents and their children“, from 2018 - “HIV / AIDS denial and alternative medicine”.
My - and not only my - opinion is that the majority of cases of non-treatment of HIV infection are caused by a banal misunderstanding that it is a controlled chronic infection, as well as by the stigmatization of people living with HIV using a common cliche, that HIV - a disease of the lower strata of society, or vice versa, the "cultural elite". This is not the case for a long time - in Russia, about 1% of the population lives with HIV, and the situation does not plan to get better.
About a year ago, several articles on this resource led me to write five notes on the history of the fight against viruses. The purpose of these articles was to describe the principles of the work of various types of drugs for HIV (the consultants were a microbiologist and an infectious diseases doctor). I hope you enjoy the transcription of these notes.
About viruses
So, a few words about viruses in general: they occupy an intermediate position between the living and inanimate world; they are incapable of independent reproduction, this requires the cells of the host body.
The virus is simple enough: it carries the genetic code, the code is closed in the capsid, the capsid is sometimes surrounded by a shell. The code can be presented in a variety of forms. The code carrier is DNA or RNA, i.e. nucleic acid (NK). There can be one and two code chains: double-stranded and single-stranded NC. The chain can be closed in a ring or linear. In 1971, David Baltimore, according to these signs, divided viruses into 7 classes. This classification is used to this day and will be important for explaining the working principles of some drugs.
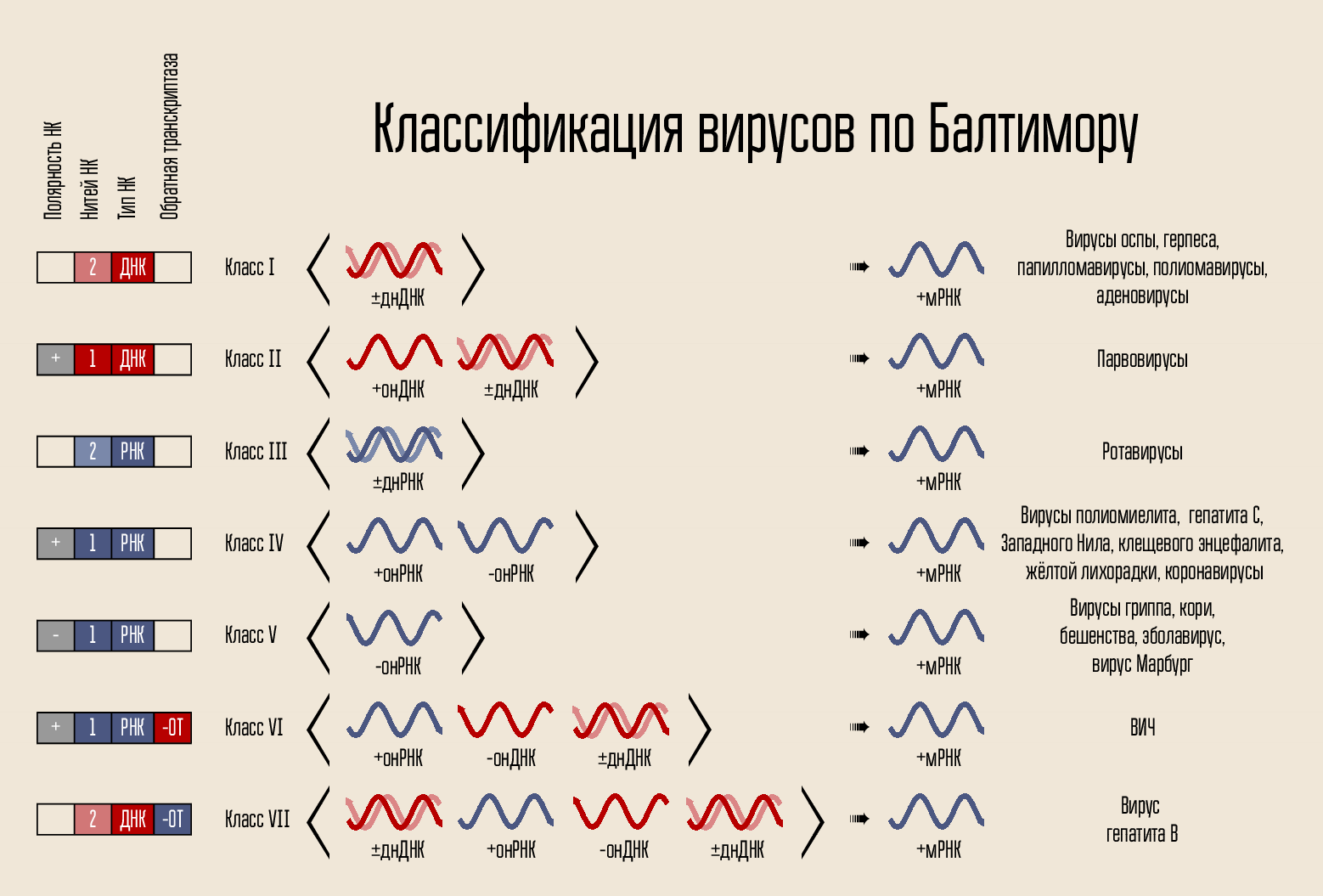
By itself, the code for building a new virus cannot penetrate the cell itself, it needs some kind of penetration mechanism. Therefore, there is a protein shell - the capsid, which protects the NK virus, and helps it enter the cell. In some cases, viruses may have additional lipid membranes.
Virus penetration into the cell
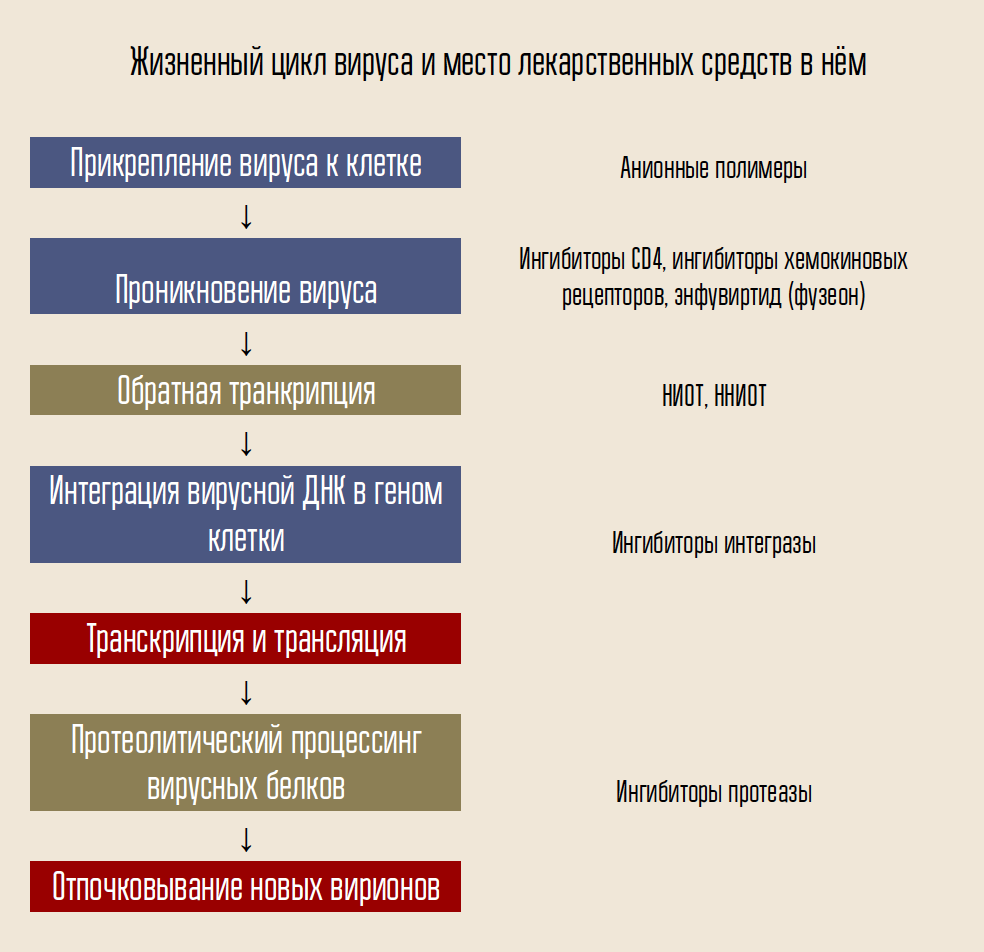
To enter a cell, the virus must connect to its shell. To do this, there are proteins on the surface of the virus that bind to the receptor proteins of the host cell - places on the surface of the cell wall to which the virus is able to attach. And they must strictly approach the virus, otherwise it will not even be able to cling to the cell.
To do this, HIV uses the CD4 receptor (cells with such a receptor are the body's immune cells, including T lymphocytes, monocytes, macrophages, etc.). CD4 alone is not enough - we need another coreceptor, either CCR5 or CXCR4. HIV uses the gp120 envelope protein to attach HIV. After that, the form of another virus coat protein, gp41, changes. He folds to the side, forming a "hairpin" and allowing the capsid of the virus to merge with the cell.
One of the agents designed to combat the virus, enfuvirtide (fuzeon), is an inhibitor of the gp41 protein. Enfuvirtide combines with this protein, preventing the formation of hairpins. Thus, the virus capsid cannot merge with the cell and infection does not occur. This drug is the only developed and approved fusion inhibitor (fusion).
Retroviruses, which include HIV, are an extremely inconvenient target for drugs because of their variability. Human cells are much less volatile. About 1% of the population of northern Europe is known to be immune to HIV: they are carriers of the CCR5-32 mutation, which makes the CCR5 receptor form unsuitable for combining with HIV.
Unfortunately, changing the form of this receptor forever, including new cells that continue to appear in the human body, is an extremely difficult task (although there have been attempts), but developing a receptor inhibitor is a drug that would join the cell receptor and thus prevent HIV from joining. to him is quite possible.
Several inhibitors of the CCR5 and CXCR4 receptors were in development, but the only one approved to date is maraviroc, a CCR5 inhibitor.
Reverse transcription
What happens after the merger of the virus with the cell in the case of HIV?
HIV is a Baltimore class VI virus; it stores its genome in RNA. There is DNA in the cell's core, so HIV needs to be turned one nucleic acid into another. Such rewriting (NK → NK) is done by the corresponding enzymes, called polymerases. For RNA-dependent (i.e., DNA polymerase reading information from RNA) (i.e., DNA appears at the output) there is a special name - reverse transcriptase. Reverse transcriptase takes the desired deoxynucleoside (for simplicity, deoxynucleoside triphosphates are actually involved) and builds DNA that is complementary (appropriate) to viral RNA.
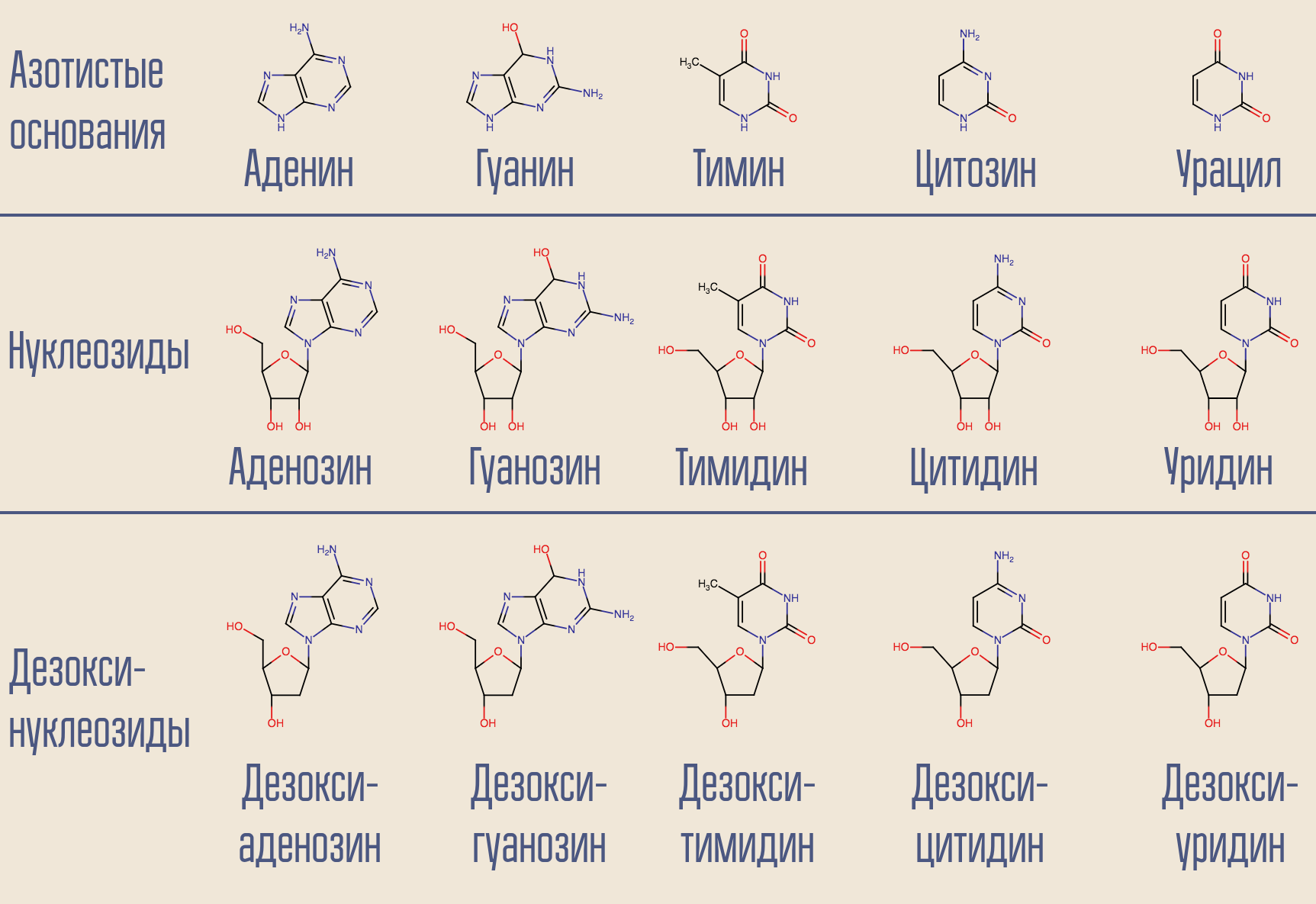
Is it possible to interrupt this process? Yes, for this you just need to slip the reverse transcriptase into something resembling but not deoxynucleoside. This is how the very first anti-HIV drug acted - zidovudine (azidothymidine, AZT). It is similar to deoxythymidine, but it is not.
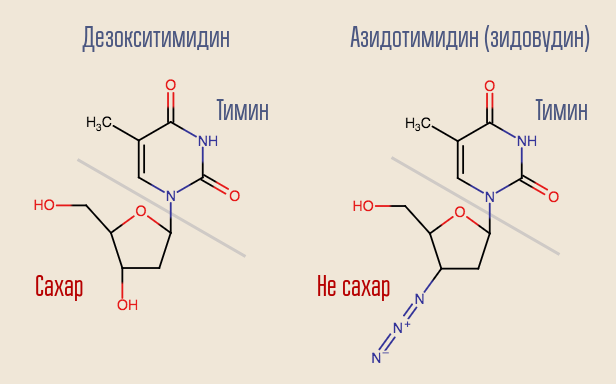
Azidothymidine was developed as part of a search for substances that could fight tumors. It was assumed that it will be built into the construction of the ordinary human DNA, interrupting it. Thus, the drug would most strongly affect the most rapidly dividing cells - tumor cells. There were certain reasons for thinking this way - another drug of this group, previously synthesized, 6-mercaptopurine, was effective in the treatment of leukemia.
Unfortunately, during the animal testing process, the drug showed its ineffectiveness and was forgotten for some time, until in 1984 virologist Marty St. Clair, who worked in the laboratories of the Burroughs Wellcome Foundation, did not initiate research to check all available substances for treatment they have a new disease - HIV infection.
Reverse transcriptase “recognized” zidovudine as deoxythymidine, and tried to integrate it into DNA. DNA synthesis at this site was interrupted, since the drug was only similar to deoxythymidine. Zidovudine completely suppressed the reproduction of the virus, and human trials were started almost immediately.
HIV-infected volunteers were divided into two groups, one of which received a placebo and the other AZT. The difference between the two groups was so striking that further testing was considered inhuman - the drug showed a staggering efficiency.
The success of zidovudine stimulated the research of other nucleoside reverse transcriptase inhibitors (NRTIs), and many other drugs appeared in a short time. The most interesting of the first drugs is lamivudine, an analogue of another deoxynucleoside, deoxycytidine. The disadvantage of lamivudine is that with monotherapy with this drug very quickly, within about a month, resistance develops. This is due to a single single point mutation of HIV, M184V. Despite this, lamivudine was desirable to leave in the scheme. The fact is that a virus with this mutation is hypersensitive to zidovudine, and the mutation itself reduces the rate of viral replication.
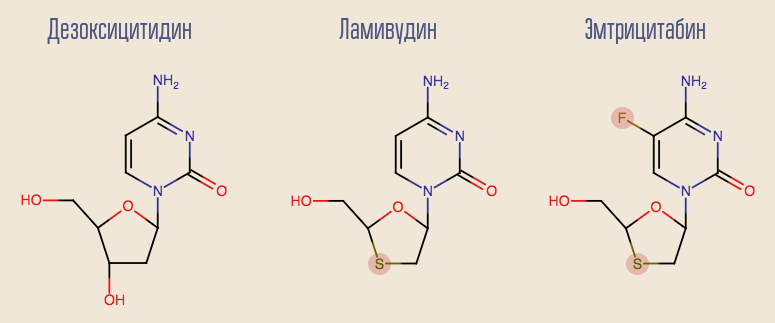
Currently, lamivudine is gradually beginning to become a thing of the past, giving way to its more modern counterpart, emtricitabine. Both lamivudine and deoxyadenosine analogue - adefovir - show good results in treating hepatitis B. Unfortunately, adefovir showed its ineffectiveness in treating HIV. However, after a small modification of its molecule, its updated version appeared - tenofovir. Tenofovir and emtricitabine are part of many modern lines of therapy.
The combination of two NRTIs could significantly prolong the lives of people living with HIV, but it was clear that to fully suppress the virus, it was necessary to include at least one drug of a different type of action, because sooner or later the virus developed resistance to any combination of NRTIs. One of the first substances of a different type of action was a different type of reverse transcriptase inhibitors — non-nucleoside (NNIOT). Although the reverse transcriptase (OT) wants to work with something similar to a nucleoside (nucleoside triphosphate), you can try to make such a substance that will bind to the OT and change its shape so that it can no longer perform its functions.
In 1996 and 1998, two such substances, nevirapine and efavirenz, were approved respectively. Each of them effectively suppresses the work of OT, and in combination with two NRTIs, it is a complete scheme of highly active antiretroviral therapy (HAART) - sufficient for a person living with HIV to live a full life not too long in life. .
In 2006, the first combination drug was approved, which is intended for a single dose per day: Atripla. Atripla consists of two NRTIs, emtricitabine and tenofovir (in the form of tenofovir disoproxil, a prodrug, a chemically modified dosage form that turns into medicine directly in the body), and one NNRTI, efavirenz. Atripla has become a qualitatively new step towards improving the quality of life of patients. Today Atripla generics are one of the most commonly used drugs in the world (in developing countries).
However, today the NNRTIs are gradually leaving the market - old drugs cause various side effects. For example, the first two months after the start of efavirenz efavirenz may cause dizziness and other similar effects in some patients (not all!). Of course, this is much better than imminent death; Such a state does not last too long, and they have already learned how to fight it - however, the current trend is the transition to such drugs, the side effects of which the patient does not notice at all.
Integration
If the reverse transcriptase did its job, is it possible to stop the introduction of viral DNA into the cell's DNA? This process is engaged in a special enzyme called integrase.
The process of integration of viral DNA proceeds in several stages. First, integrase binds to viral DNA, removing the dinucleotide GT from the 3'-end of each strand. Then the whole complex is transported to the nucleus, where integrase catalyzes the chain transfer stage. This stage is a transesterification reaction (radical exchange): the DNA nucleotides of the cell become connected not with each other, but with the nucleotides of viral DNA. Integraza attacks internucleotide bonds located at a distance of five nucleotides. Thus, after integration, the processing of the 5'-ends of the viral DNA chains, the completion of 5 missing nucleotides and ligation (the connection of two strands of NK by a ligase enzyme), which are performed with the participation of cellular proteins, remain [1].
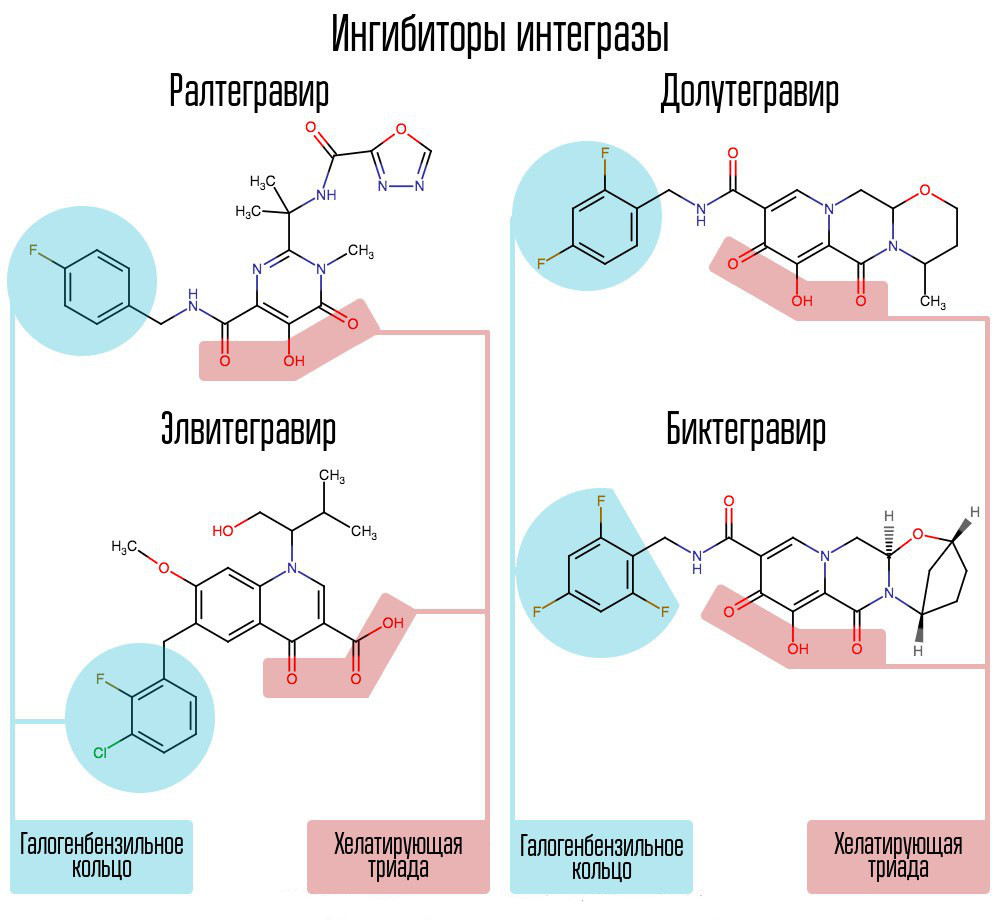
Screening of about 250000 substances in chemical compound libraries made it possible to find substances that would inhibit HIV integrase. All of them were compounds of 2,4-dioxobutanoic acid. They coordinated the metal ions in the active center of integrase - in the part that was responsible for the transfer of the chain. Further attempts to develop HIV-1 integrase inhibitors led to the emergence of a derivative of N-pyrimidinone, a substance MK-0518, called raltegravir. [2]
Common to raltegravir and subsequent integrase inhibitors are a chelating triad (coordinating metal ions) and a halobenzyl ring that interacts with the penultimate deoxycytosine at the 3 ′ end associated with viral DNA enzyme.
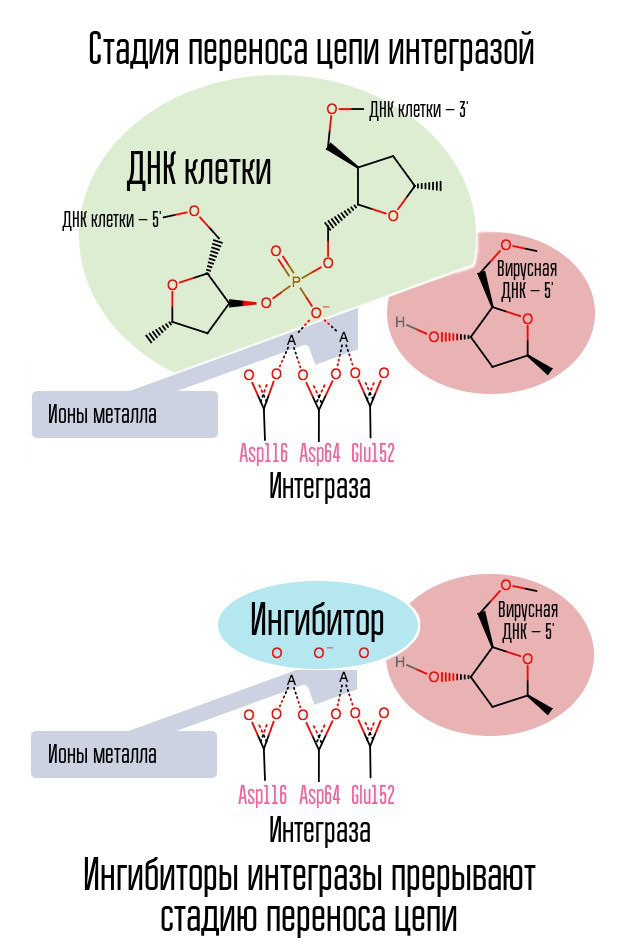
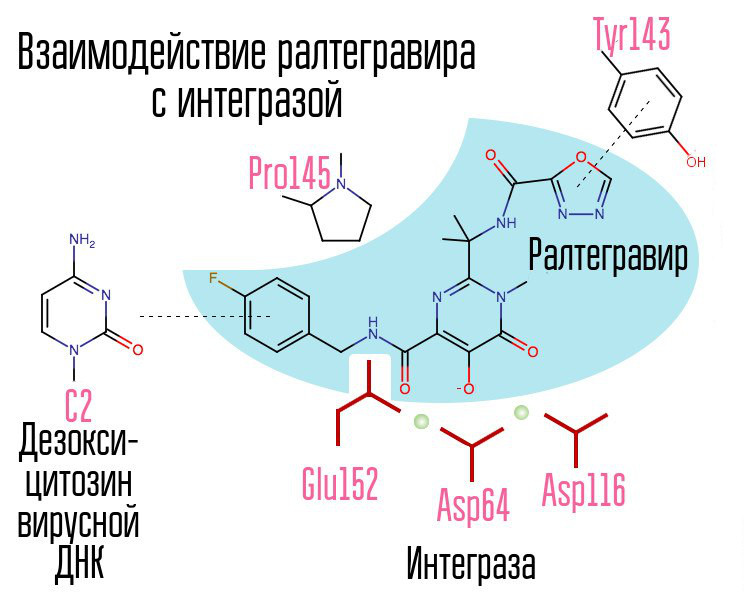
The process of integrating the virus into the cell is the last stage at which post-exposure prophylaxis is effective. After that, cells appear in the human body that carry HIV in their nucleus. The most effective window for post-exposure prophylaxis is about 6-10 hours.
A halogen-benzyl ring in the integrase inhibitor molecule interacts with viral DNA, and a group of oxygen atoms interacts with two metal atoms. These metal atoms integrase virus uses in order to introduce viral DNA into the cell. As a result, the integration process is blocked.
Modern AIs, such as dolutegravir, were able to defeat the childhood diseases of raltegravir, associated with the rapid formation of resistance.
Proteolysis
After the viral genome passes the transcription stage, the viral RNAs created are directed to the exit of the cell. In the process of creating the virion, another viral enzyme, called protease, is involved. Protease cuts long polyproteins into individual functional proteins, resulting in the formation of viral enzymes and structural proteins of the virus.
Protease is active not only against HIV proteins, but also against proteins of the host cell, which may explain the cytotoxic effect of HIV (cell death).
If you block the protease, the virion will not be able to pass the stage of maturation and will remain completely non-functional. HIV-1 protease is a typical retroviral aspartate protease, having the characteristic amino acid sequence Asp25 Thr26 Gly27 (aspartic acid - threonine - glycine) in the active center. The first protease inhibitor, saquinavir, was approved by the FDA on December 6, 1995. Thus, it was after the creation of saquinavir that highly active antiretroviral therapy became available for the first time.
Another typical representative of this group of drugs is lopinavir (used in conjunction with ritonavir - kaletra is one of the most common HIV drugs in Russia). Ritonavir is also a protease inhibitor, but is used as a booster - due to its action, the concentration of the main drug increases.
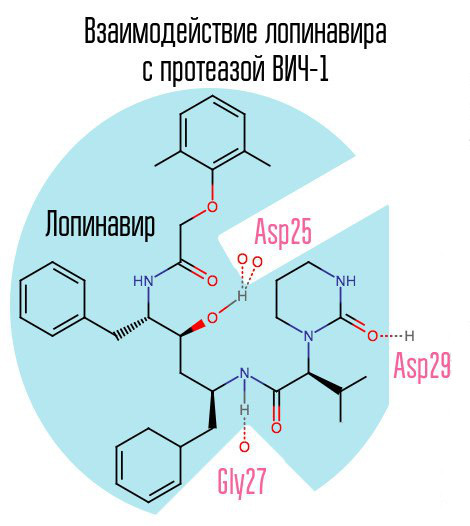
Since saquinavir and the subsequent protease inhibitors (PIs) are aimed specifically at the active center of the enzyme, with the development of resistance to one PI, there is a high probability that resistance will occur to other PIs. The solution to this problem may be the creation of such inhibitors that are directed to other protease zones.
The drug darunavir (prezista), which appeared in 2006, somewhat relieved the problem of PI-resistant strains of HIV-1, as it formed a previously unused connection with aspartic acid at position 30.
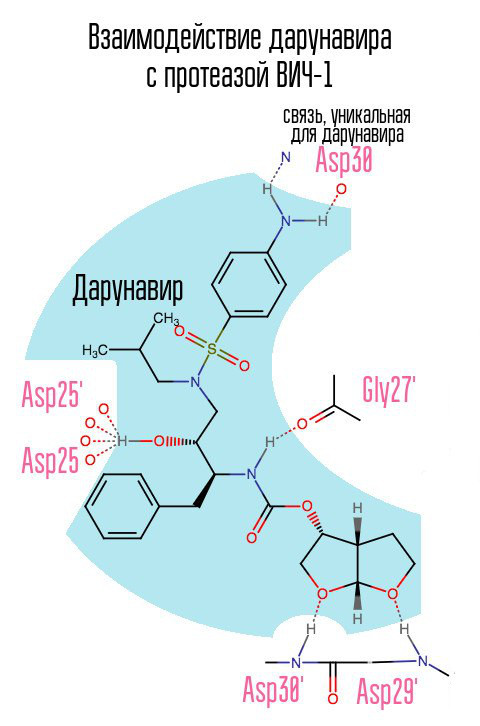
Without protease, the virus cannot mature. Inhibitors are connected to the active center of the protease and do not allow it to work.
Protease inhibitors are highly effective means with a high viral load: since at this point many new virions are born in the body, PIs do not allow them to mature, thus effectively reducing the viral load in a short time. However, at the moment PIs are not used in first line therapy, giving way to integrase inhibitors (AI).
The reason for this was side effects: the fact is that, for example, the same kaletra led to nonspecific inhibition of proteolysis of food proteins, as a result, these proteins entered the small intestine and caused diarrhea. Observance of a specific diet or the use of new PIs, such as the prezista, makes it possible to reduce this effect to almost zero, but another effect associated with an increase in the level of sugar often does not allow the use of protease inhibitors indefinitely.
Modern treatment regimens
Today, the most up-to-date are the schemes consisting of an integrase inhibitor and one or two NRTIs (Dolutegravir + Abacavir + Lamivudine; Dutegravir + Lamivudine - a popular two-component scheme, suitable, however, not for everyone). These schemes allow a person to live a full life, not differing in duration from the life of a person without HIV.
Despite all the successes, a complete recovery from HIV is still impossible (transplanting bone marrow stem cells from a donor with a mutation CCR5-∂32 allows you to achieve this result, but, apparently, only if a graft versus host disease occurs, in a large number of cases leading to the death of the recipient).
Conclusion
Developed methods of combating HIV helped in the fight against other infectious diseases: as mentioned above, lamivudine and tenofovir are effective against the hepatitis B virus (Baltimore class VII - Hepatitis B polymerase is able to rewrite RNA in DNA, therefore some NRTIs are effective in fighting it) . The knowledge gained has helped to develop drugs of direct action against hepatitis C, which today make it possible to completely cure this disease (hepatitis C does not have a latent phase, therefore, when viral load is suppressed, there is no place for new virions - the disease is completely cured).
[1] Korolev S. P., Agapkina Yu. Yu., Gottikh M. B. Problems and prospects for the clinical use of inhibitors of HIV-1 integration
[2] V. Shahgildyan. HIV integrase inhibitors - the basis of effective and safe antiretroviral therapy
')
Source: https://habr.com/ru/post/449336/
All Articles