Inside the flame: a new method for studying high-temperature reactive systems

In ancient Greek mythology, a special place is occupied by a character who zealously defended humanity from the cruelty and arbitrariness of the gods. Among other things, he gave us, the people, the fire and the knowledge of how to keep it. The name of this character is Prometheus. Zeus punished him in the most cruel and sophisticated manner - Prometheus was chained to the rock for ever and ever, and the eagle every day pecked out his liver, which completely regenerated, and the agony repeated again. Not all physical or chemical phenomena get their own mythology, but fire is another matter. Life-giving and at the same time destroying everything in its path, so simple and so mysterious. Today we will get acquainted with the work in which scientists have demonstrated a new method of exploring fire, which allows for a closer look at molecular processes occurring in flames. What tools and tools were used by scientists, what new things could they learn about fire and how can their work help humanity in the future? Answers are always waiting for us in the report of the research group. Go.
The basis of the study
Sometimes, looking at the flames, it seems that this is a living creature with its own thoughts and insidious plans. However, the mythical flame as much as enough of our imagination. In reality, fire is the same physical and chemical process as crystallization of water, for example. Fire is an oxidation process, which is accompanied by radiation in the visible range and the release of heat, that is, thermal energy. For the existence of fire requires certain ingredients: fuel, oxidizer and temperature. Imagine the most common bonfire in the camp of tourists. Wood serves as a fuel, and oxygen, which is present in the air surrounding tourists, and, naturally, wood for a fire acts as an oxidizing agent. Without oxygen (i.e., an oxidizing agent in a broader sense), the combustion process is impossible. The third ingredient, temperature, is determined by the properties of the previous two. There are many variations of each of the constituent elements of the fire, as well as their combinations, each of which has its own properties, characteristics and distinctive features. We have quite a lot of knowledge about the combustion process, but not all.
')
In the study considered today, the scientists decided to measure the temperature of the fire at various input variables: the temperature range is 1000–1800 K, the pressure is 2.0–2.9 at and 7.6–10.7 at, the frequency is 250 kHz. For this, a quantum cascade laser (hereinafter QCL) with acoustic-optical modulation (hereinafter AOM) was used with an average infrared range of the output signal from 1975 to 2260 cm -1 .
Scientists note that laser absorption spectrometry in the mid-infrared region is excellent for temporal measurements of non-intrusive particles in reactive systems. Comparison of the absorption forces of two target particles with different temperature dependences is already a method of two-line thermometry. In this method, due to limitations in scanning speed and wavelength range, it is necessary to use several lasers simultaneously for faster measurements. In addition, despite the sensitivity of measurements in low-concentration media, narrow-band lasers are not suitable for systems with a high concentration of target particles.
Thus, this method cannot be used for temperature measurements in systems of energy-intensive materials, such as C 4 H 8 N 8 O 8 (octogen) and C 3 H 6 N 6 O 6 (hexogen), since they contain target elements (H 2 O , CO, etc.) are produced in very high concentrations. Therefore, a new method of researching such systems is needed, which scientists describe in their work.
Preparation of the experimental setup
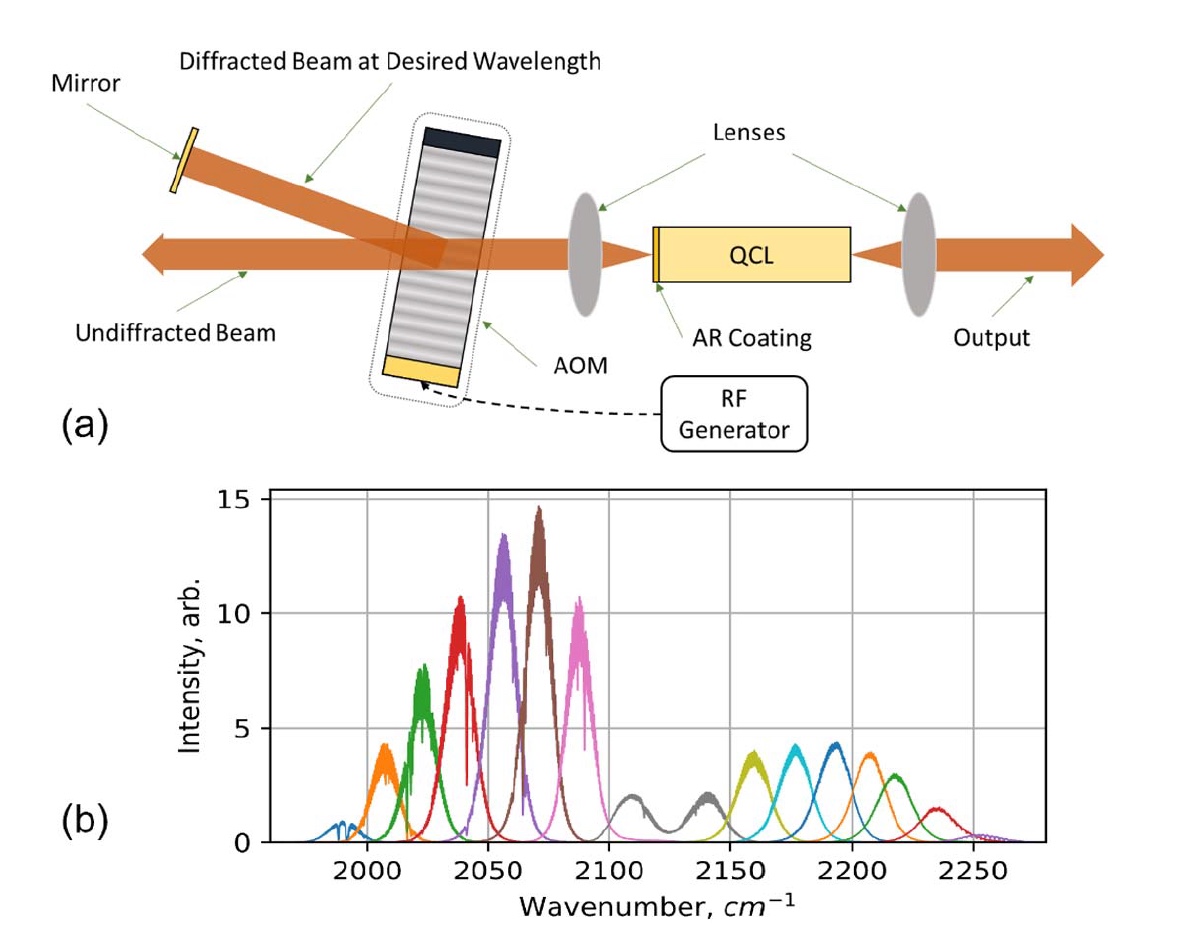
Image number 1
Image 1a shows the experimental setup of a quantum cascade laser with acoustic-optical modulation (AOM QCL):
- Mirror - a mirror;
- Diffracted Beam at Desired Wavelength - the reflected beam at the desired wavelength;
- Undiffracted Beam - unreflected beam;
- AOM - acoustic-optical modulator;
- Lenses - lenses;
- QCL -quantum cascade laser;
- AR Coating - antireflection layer;
- RF Generator - radio frequency generator;
- Output - output signal.
The spectral output of the AOM QCL was measured as a function of the input AOM RF using Fourier transform infrared radiation with a spectral resolution of 0.2 cm -1 ( 1b ).
The half width of the output signal depends on the operating conditions: the pulse duration and the QCL and AOM frequencies. In this experiment, the half-width indicator was about 12–15 cm -1 .
In experiments, a shock tube made of stainless steel with an inner diameter of 14 cm was used, polycarbonate diaphragms 0.18 and 0.76 mm thick were also used. Five piezoelectric pressure transducers located along the last 1.4 m of the shock tube were used to measure the impact velocity, which was linearly extrapolated to the end wall. The temperature and pressure in the reflected impact area (P5 and T5) were calculated using the initial temperature and pressure in this area and the extrapolated impact velocity using one-dimensional impact ratios, taking into account chemically frozen, vibrationally balanced gases. The damping rate was about 1.5% / m, and the error in T5 and P5 was less than 2%.

Image number 2: the scheme of the experimental setup in conjunction with the test area of the shock tube.
Explanation of the image above:
- AOM QCL System - installation of a quantum-cascade laser with acoustic-optical modulation;
- RF Generator - radio frequency generator;
- Function Generator - a function generator $
- Sync - synchronization;
- Pulsed Current Source - pulsed current source;
- Iris - diaphragm;
- I (transmitted signal intensity) Detector - transducer signal intensity (I)
- Curved Mirror - a curved mirror;
- I0 Detector - reference beam intensity sensor;
- Beamsplitter - beam splitter;
- Mirror - a mirror;
- Endwall - end wall;
- ZnSe Windows - zinc selenide lenses;
- Shock Tube - shock tube.
The beam from AOM QCL was divided into reference and signal beams by means of a calcium fluoride (CaF 2 ) beam splitter. The intensity of the reference beam was measured using a photoelectric sensor with thermoelectric cooling. As we see according to the scheme, in front of the tube itself the beam passes the lens from zinc selenide 3 mm thick and 12.7 mm in diameter. Both lenses were located at a distance of 2 cm from the end wall and were aimed at each other. Having passed the second lens, the beam is directed to the sensor of the intensity of the transmitted signal through a curved mirror.
The setup laser operated in a pulsed mode with a repetition rate of 500 kHz and a pulse duration of 100 ns. AOM was used to alternate pulses between 2030 cm -1 and 2080 cm -1 spectral bands by modulating the RF driver with a square wave * with a period of 250 kHz, which was synchronized with the laser pulse driver.
Meander * - a rectangular periodic signal.Such accurate spectral bands were chosen specifically to provide high temperature sensitivity in the temperature range under study with relatively low sensitivity to the mole fraction and pressure of CO. In addition, for more spectral stability, AOM and QCL were temperature controlled.
Experimental results
Now let's go directly to the results of the installation.
During the experiments, the measured temperature was varied from 1000 to 1800 K, with two types of pressure applied: low - 2.0-2.9 atm and high - 7.6-10.7 at. The analyzed mixture consisted of CO, diluted in helium (He) and argon (Ar). At low pressure, a mixture of 10% CO, 25% He, and 65% Ar was used, and at high pressure, a mixture of 3% CO, 15% He, and 82% Ar was used. To ensure the homogeneity (uniformity) of the samples, the mixing process proceeded for 8 hours.

Image number 3
To calculate the expected spectrum of the signal beam for each of the two wavelength bands, we used a set of simulated absorption spectrum and the measured output spectrum of the AOM QCL. The monochromatic Beer-Lambert law was taken into account for each individual wavelength ( 3a ).
Absorption for two bands was modeled for each combination of T5, P5 and molar fraction with 14 cm of track (shock tube length) taken into account for: temperature range of 600-2600 K with a step of 50 K, range of mole fractions of CO from 1% to 50% with a step 1% and a pressure range of 0.001-13.0 atm with 1 at.
As can be seen in image 3b , temperature strongly influences the absorption coefficient, but weakly on the mole fraction and pressure. The temperature and molar fraction were calculated using the iterative method, that is, these indicators were first determined separately from each other and used to calculate the theoretically expected absorption values for the two output bands at the experimentally measured pressure (P5). After that, the temperature was changed by comparing the theoretical and measured absorption coefficients. The molar fraction of CO was changed using the difference between the measured and theoretical absorption values of the band at 2080 cm -1 .
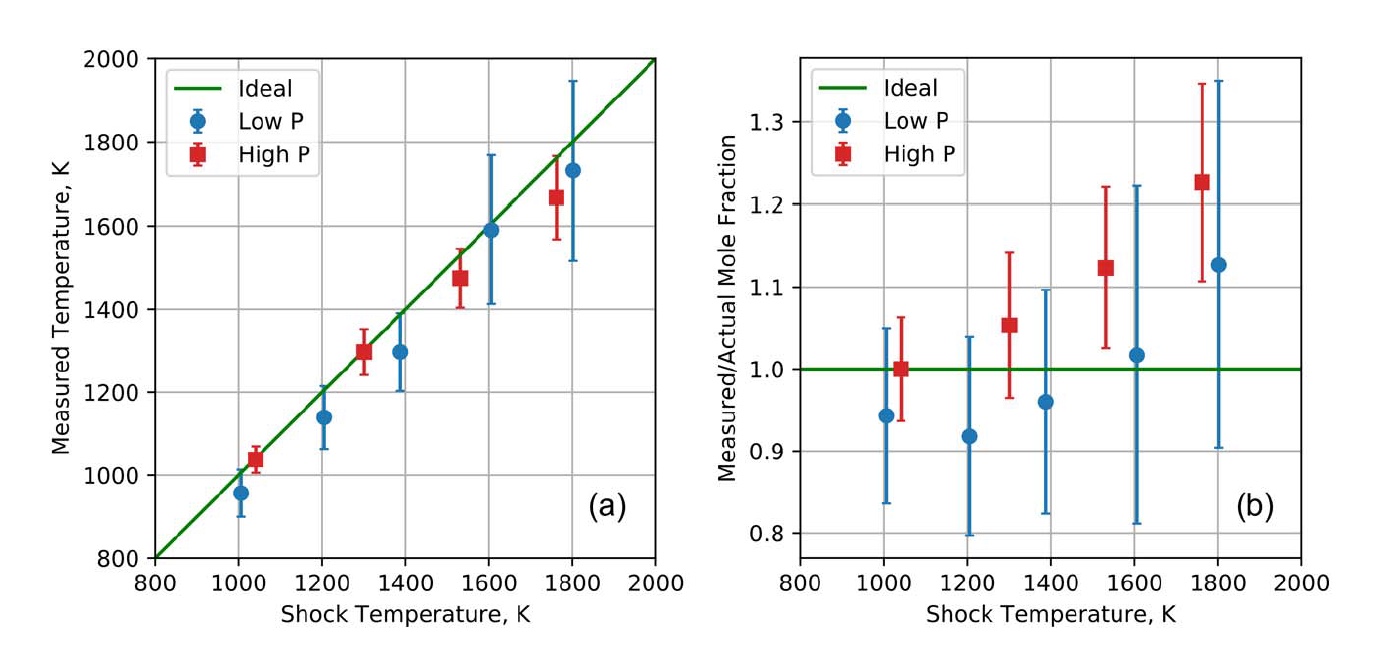
Image number 4
In image 4a, we can see a comparison of the measured and known temperatures in the experiments with a shock tube, taking into account the impacts of both low and high pressure. As we can see, the temperatures that were obtained by means of the AOM QCL system are almost perfectly coordinated with the values of impact temperatures in the entire range of 1000–1800 K and for both pressure ranges. The adjacent graph ( 4b ) shows the ratio of the measured and actual mole fraction of CO. In the case of this indicator, there is also an excellent agreement between the initially known data and those obtained by measuring with the experimental AOM QCL system.
The findings of researchers
Scientists have discovered that the temperature change does not depend on the displacements of the laser beam due to the particles of the diaphragm. This conclusion is justified by the fact that this offset affects both spectral components of the output signal due to the variable pulses passing through the same beam. As a result, the offset is compensated.
If we take into account the immunity of temperature measurements to background thermal emissions and the above-described laser beam offset, then the developed method is excellent for studying gas-phase reactions of energetic materials (for example, HMX and RDX) in which CO is formed, and hot particles and pressure waves can cause both thermal outliers and beam offset.
In addition, given the AOM QCL bandwidth of 12-15 cm -1 , many of the absorption characteristics of one component of the medium under study can be analyzed simultaneously. Narrow-band lasers, on the other hand, have an increased sensitivity, but are limited in the concentration range where they can be used, due to saturation.
Spectrum modeling using HITEMP considers CO only. Accordingly, the use of the AOM QCL system on structures, when the component components of the mixture can be different, requires further improvement of the system to improve its accuracy.
For more detailed acquaintance with the nuances of the study I strongly recommend to look into the report of scientists .
Epilogue
This experimental study demonstrates a new instrument in the study of temperature and component concentration inside high-temperature reactive systems. Scientists with this tool were able to successfully study mixtures with 3% and 10% CO in the temperature range of 1000 ... 1800 K at a pressure of 2.0-2.9 atm and 7.6-10.7 at.
The AOM QCL system, according to the developers themselves, is quite flexible and allows you to configure it for different test environments in a wide temperature range. In addition, the system can measure several components of the medium at once by measuring their absorption characteristics.
Fire is not just a stove in a village house, a fireplace in a mansion or a candle on a cake. Fire is a complex physico-chemical process, the understanding of which gives a person more tools to control his creative power and fight against his destructive power.
I will not exaggerate saying that we were all shocked by the fire that occurred in the Cathedral of Notre Dame. So many centuries of scientific research, discoveries and breakthroughs, but we could not save one of the greatest and most beautiful pearls of architecture from unruly fire. This loss once again reminded us that man is so great, and we still have a lot to learn about the world around us, in order to fully protect ourselves from the misfortunes that he can teach us. The only destructive force with which we most likely will never be able to cope is ourselves.
Thank you for your attention, remain curious, remember the rules of fire safety and a good work week for you guys.
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to friends, 30% discount for Habr's users on a unique analogue of the entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to share the server? (Options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps before summer for free if you pay for a period of six months, you can order here .
Dell R730xd 2 times cheaper? Only we have 2 x Intel Dodeca-Core Xeon E5-2650v4 128GB DDR4 6x480GB SSD 1Gbps 100 TV from $ 249 in the Netherlands and the USA! Read about How to build an infrastructure building. class c using servers Dell R730xd E5-2650 v4 worth 9000 euros for a penny?
Source: https://habr.com/ru/post/449086/
All Articles