About simple things, difficult. Chemist writing to a 3D printer. Solvents for plastics and protection against them
DIY is dedicated to ...
One of the most frequently asked questions in my consulting practice is issues related to the dissolution / bonding of plastics using various organic solvents. Recently, there has been a real surge of interest in the chemistry of high-molecular compounds, associated with the advent of affordable 3D printers and the need to navigate in the “ink” for them (ie, polymer filaments, filaments). Once again, I am convinced that not even the most advanced “science museum” with a spectacular show can make IT-person more interested in plastics than his own 3D printer. So, reader, if you ever had to think about how to glue plastic, which the default superglue did not glue, if you had doubts about the dissolution of the freshly printed part supports, and it’s just interesting how you can clean the glue from the store price tag on the gift - I ask cat I also strongly recommend that the page be bookmarked not only by those who are often involved in gluing plastics, but also to all those who often have to work with various solvents / thinners. It was done for myself - donated to Habra!

As I wrote a couple of times in the comments to my articles, lately, from time to time I have an idea to make myself an “exhibition” stand, on which samples of plastics would be presented. Just because almost every second chemical question sounds “what kind of plastic is this.” What it says, says that the possibilities of 3D printing have attracted such public attention to plastics, polymers, etc. which hundreds of online popularizers could not have done. Well, in general, looking at these trends, we can safely say that the future, the future is not so much for metals, as for composites and new types of polymers. So, the one who today is thinking about the choice of chemical specialty - consider this option. Therefore, once again, your humble servant decided to make his modest contribution and talk about what I constantly encounter. Today we read about solvents for plastics and the features of working with them. For a start, a little theoretical introduction.
')
To tell in a few words about the dissolution of polymers will not work with all the desire, because the topic is voluminous and ambiguous (you can even say “pull on a university course”, hello to you, Leonid Petrovich Krul , I pay the debt for 8 on the Navy). A good (read educational) review for people with a fairly high level of technical (chemists and engineers) literacy can be read here . The process of dissolution will be discussed below, in the meantime, a few words about the choice of solvent (or why something dissolves plastic, but something does not).
In general, the selection of a suitable solvent is made by two methods:
1. Using the Hildebrand solubility parameters . This calculation is used if the polymer (p) and solvent (s) have the same polar and hydrogen bond parameter, then the following simple rule works:
| δ s - δ p | ≤ 3.6 MPa 1/2
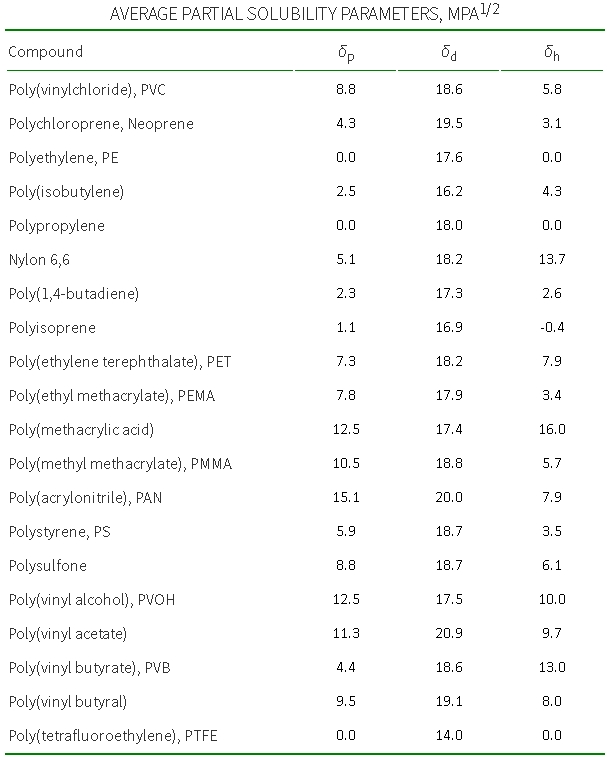
As an example, I will give the Hildebrand parameters for some polymers:

Who wants to check himself - can calculate solubility at his leisure :). Search for constants can and should be here in this book. It is important to note that the Hildebrand parameters are only useful for non-polar and low-polar mixtures in the absence of hydrogen bonds (dipole moment <2 D (Debye)). For other cases, method 2 is used.
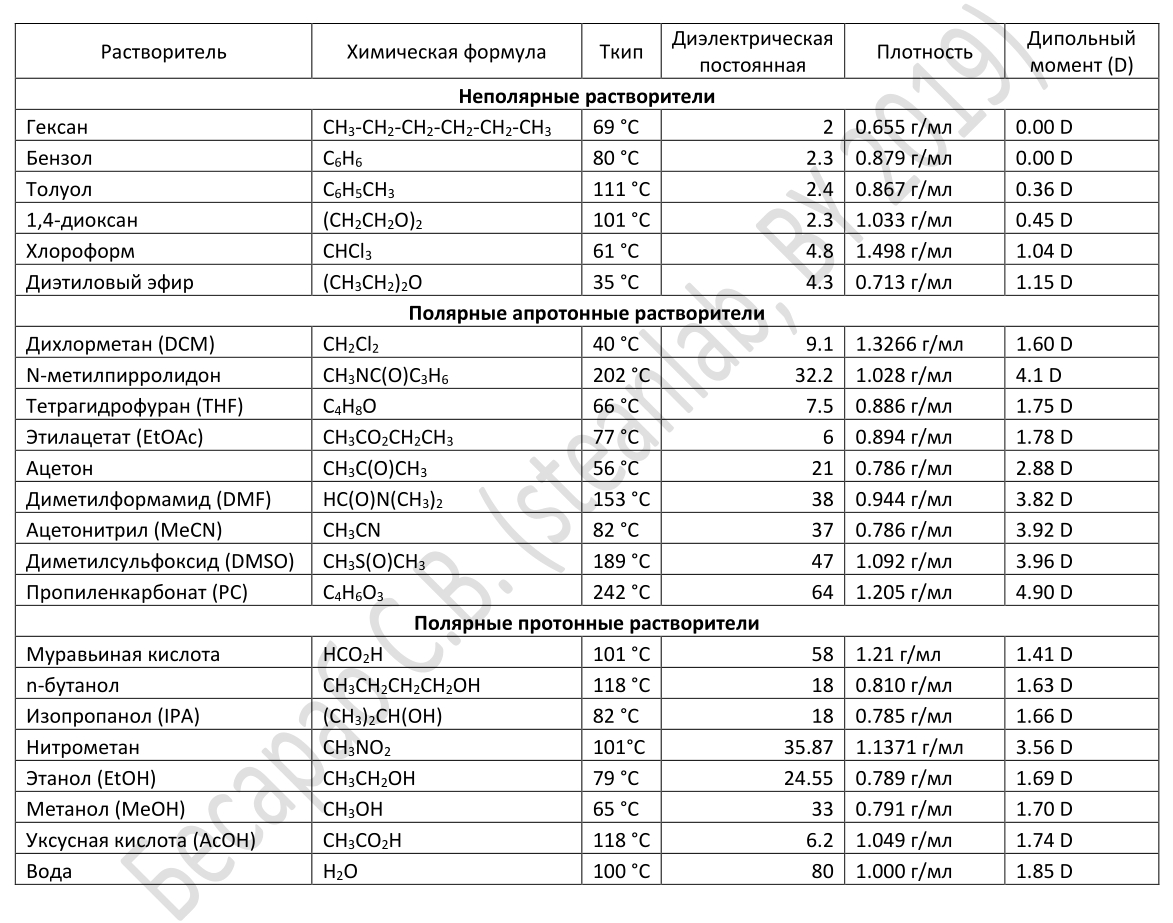
Note: for those who traditionally “knew, but forgot”, I remind you that according to the IUPAC standards (what they look for in the article about the Periodic Table ), solvents are qualitatively grouped into non-polar, polar aprotic and polar proton solvents, for dividing into groups their dielectric constant is often used. Most often, a proton solvent is a solvent that has a hydrogen atom bound to oxygen (as in a hydroxyl group), nitrogen (as in an amino group), or fluorine (as in hydrogen fluoride). In general, any solvent that contains mobile H + is called a proton solvent. Molecules of such solvents easily give protons (H + ) to other reagents. Conversely, protons cannot give aprotic solvents, since they do not contain H + . They usually have high dielectric constant and high polarity. The picture below shows examples of common solvents, divided into classes.
We return to the selection of solvent. As I already wrote, if Hilderbrant did not fit, we use Hansen.
2. Using the Hansen solubility parameters, for each solute one can make an approximate spherical “volume” of solubility with radius R. Only solvents that have Hansen solubility parameters in this volume can dissolve this polymer:
[4 (δ d2 - δ d1 ) 2 + (δ p2 - δ p1 ) 2 + (δ h2 - δ h1 ) 2 ] 1/2 ≤ R
The radius of interaction R depends on the type of polymer. R values are usually in the range of 4 to 15 MPa 1/2 . The parameters of Hansen needed to calculate the solubility of your system can be found in this book . For clarity, the picture below shows the parameters of Hansen (by analogy with Hilderbrant) for some widely used polymers.
If all of a sudden someone really needs to carry out a targeted solvent screening for their polymer using the Hansen method, I recommend paying attention to the HSPiP program, which does an excellent job with this task. Under the link - an overview and description of the work.
In general, we can say the following. First, the “golden rule of dissolution” - like dissolve in like - works for polymers. Those. Compounds with a similar chemical structure are more prone to dissolution than compounds with different structures. Secondly, the higher the molecular weight of the polymer, the closer must be the solubility parameter of the solvent and the polymer for the polymer to dissolve in the solvent. For linear and branched polymers, the graph of solubility versus solubility parameter for a number of solvents reaches a maximum when the solubility parameters (Hansen / Hilderbrandt) of the solute and solvent coincide. In the case of a crosslinked polymer, the volume of swelling, i.e. the absorption of the solvent, will reach a maximum when the solubility parameters of the solvent coincide with those of the polymer. Third, the solubility parameters of polymers do not change much with temperature, while parameters of low molecular weight compounds often decrease markedly with increasing temperature, so the higher the molecular weight of the polymer, the closer the solubility parameter of the solvent must be to dissolve the polymer in the solvent.
Okay, I hope the reader is not tired. I hasten to move from theory to practice.
Traditionally, if you suddenly need to grow several pieces of plastic using different methods. Some of them are shown in the picture:

In industry, either welding based on physical methods (such as ultrasonic or laser) or mechanical coupling is often used. Adhesion methods of bonding (glues, melts or polymer solutions) are much less commonly used. Such methods are used in the assembly of plastic windows in stores, gluing together various aquariums, wardrobe trunks and covers. But probably the most popular user of this method is DIY-er, or in our opinion, a homemade man. Ever since the days of the USSR, inventors and simply handy citizens of all stripes glued the hulls of their Plexiglas and dichloroethane articles. With the advent of affordable 3D printers in our life, polymer solutions have received a second life in the form of props, which are created during printing and which need to be somehow removed in the finished product. It is not always possible (and advisable) to do it mechanically, so often his majesty the Solvent of Plastics comes into play.
Note : if you speak for yourself, despite the possibility to print a model on a 3D printer, I still use the old-fashioned glue on plexiglass when I need to make a box or something like that (without Bezier curves). At the KDPV, by the way, an example of such a “momentary!” Is shown. things ", which in haste was glued with a red plexiglass solution (PMMA) from a cone.
So, chemical plastic welding is the process of combining plastic-softened plastic surfaces. The solvent temporarily converts the polymer to a “rarefied” state at room temperature. When this happens, the polymer chains can move freely in the liquid and can be mixed with other similarly dissolved chains. After some time, the solvent due to diffusion and evaporation will penetrate through the polymer and migrate into the environment, and the polymer chains will be compacted (~ packed) and lose their mobility. A frozen ball of tangled polymer chains is a weld with this type of welding. Graphically, the mechanism of the plastic dissolution process is shown in the picture below:

Normally, a normal dissolution includes a solvent penetration stage, a polymer swelling stage, and a polymer diffusion stage into a solvent. The initially glassy polymer contains a multitude of microchannels and holes of molecular size (per so-called infiltration layer).
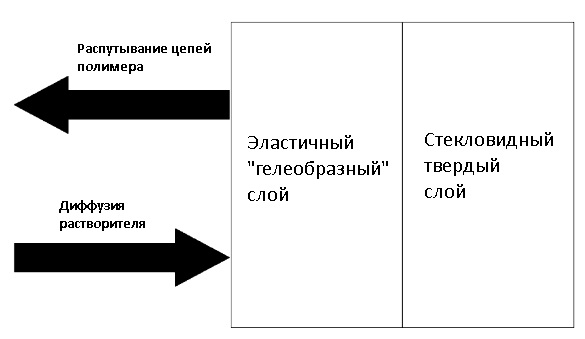
Upon contact with the solvent, the latter fills these channels and holes and starts the diffusion process (new channels are not formed). Schematically, such a surface layer of a dissolving polymer looks like this (roughly speaking, “glue” = gelatinous mass, which is in the middle between a solid polymer and a liquid solvent):

With the mechanism, I hope, everything is more or less clear, it is time to move on to the specifics of what and what. In the theoretical part, I briefly tried to explain how the solvent screening process takes place for a particular type of polymer. Those. universal and comprehensive table for the dissolution of polymers yet.
And this topic is relevant. Confirmation is the fact that quite often on the pages of various thematic resources (DIY, 3D, amateur radio, etc.) with a noticeable periodicity, there are questions like "how to handle" / "what to glue" / "how to dissolve" this or that type of plastic . It is interesting that in most cases the answers are given by people with the chemistry of polymers (IUDs) who are familiar to me, apparently rather weakly. As a result, there is even more confusion and "space for creativity" of all kinds of amateurs, vendors and other obscurantism. They lose money and time, traditionally, innocent users. So, we look at the table below and we shake on a mustache.

The dark square in the table at the intersection of the "polymer" - "solvent" lines indicates that it is possible to carry out chemical welding using these components. Note : the square at the intersection of "ABS" - "acetone" - with the letter H , because it was the Habra community that convinced me that ABS glues mainly with acetone (I dissolved ABS with acetone, but then I couldn’t glue this solution, for crumbled).
If, as a rule, there are no problems with the issue of plastic, then quite often there is a problem with the presence of the required solvent. Everyone gets to the best of their abilities - someone just orders the necessary solvents, someone searches for them at the flea market, well, and someone is trying to empirically choose from what is sold in stores. Under the spoiler, if that, the composition of commercially available solvents for varnishes and paints (taken from the chemister ).
To the note: I ’ll add a few words about polymers that are not in the table. Of course, this is the favorite "popular" filament - PLA , which dissolves best in polar aprotic solvents : pyridine, N-methylpyrrolidone, ethyl acetate, propylene carbonate, dioxane, dioxane, dichloromethane, chloroform, acetone (?? - depends on the manufacturer of the PLA-filament and contained within “additives”, the same is true for other polymers), nitrobenzene, acetonitrile, dimethylacetamide, etc. A promising 3D polymer PEEK (also known as polyetherketone) is remarkably soluble in 4-chlorophenol (a more rigid variant is a mixture of 80% chloroform and 20% dichloroacetic acid). Chlorophenols (not only 4-, but also 2-chlorophenol) can also dissolve widely distributed and hotly beloved PET . At the request of readers, I will mention a rather new polymer of the PET series, the so-called PETG (polyethylene terephthalate glycol). Like the elder brother, this polymer is resistant to a number of widely available components, it is soluble only in HFIP (hexafluoropropanol). Soft and malleable TPU (thermoplastic polyurethane), like other polyurethanes, can be dissolved in N, N-dimethylformamide (DMF), tetrahydrofuran, ethyl acetate, cyclohexanone, dimethylacetamide. By the way, polyurethane foam, it is also polyurethane. I didn’t see what was in the composition of special liquids for flushing guns for polyurethane foam, but I suspect that some of the mentioned components are definitely there. PCL polymer (polycaprolactone) is dissolved in anisole , 2,2,2-trifluoroethanol, N, N-dimethylformamide, methylpyrrolidone, tetrahydrofuran, dichloromethane, acetone, chloroform and DMSO (dimethyl sulfoxide, which is also sold in the pharmacy "Dimexide"). PDMS (polydimethylsiloxane) widely used for prototyping (especially in scientific institutions related to micro- and nanofluidics) is dissolved using glacial acetic acid. By the way, many other silicones have similar properties, starting from a two-component construction and ending with those on which stickers are glued with prices (therefore, to wash off the adhesive residue from the price tag with ABS plastic, for example, it will be most productive with some acetic acid). Well, in the end a little exotic. EVA (ethylene vinyl acetate), PP (polypropylene), PE (polyethylene, LD / HD) are dissolved in 1,2,4-trichlorobenzene, and PVP (polyvinylpyrrolidone) - in dimethylacetamide.
Since solvents, to put it mildly, this is not the scent of cherry blossoms for you, there is a safety issue when working with them. It is sad to see how young guys sometimes work without acetone, with chloroform, and even with benzene. And the rules of TB, as you know, are “written in blood” ...
The main ways in which solvents enter the human body (and their vapors) are through the respiratory organs and through the skin. I don’t consider any deviations (like ingestion), because a person in their right mind will never drink benzene. The above reagents have a predominantly narcotic effect, have a pronounced irritant effect on the mucous membranes of the upper respiratory tract and conjunctiva of the eye, moderate - on the skin. The best protection against them is to work in the conditions of supply and exhaust ventilation, in special boxes. If it happens in specialized workshops or laboratories, then most often there is already a fume hood .

If it is impossible to arrange the necessary ventilation, working with organic solvents provide personal protective equipment: respirators, gas masks, oxygen-insulating devices, etc. (depending on the vapor concentration). In general, solvent vapors are remarkably sorbed by activated carbon (and many other sorbents), not without reason, some solvents were actively used to assess the sorption capacity of a material (the so-called “exicator method”). I personally “had the honor” to check the sorption capacity of coals by their absorption of carbon tetrachloride CCl 4 . Most of the vapor can hold a gas mask with a class A box or a respirator mask with a similar filter cartridge. Like this:

It is important in the description to look for something like " protects against vapors of organic compounds (gasoline, kerosene, acetone, benzene and its homologues, xylene, carbon disulfide, etc.), phosphorus and organochlorine toxic chemicals, dust, smoke, fog ." But to such a mask, it is also desirable to have sealed glasses, whose glasses are rubbed with fogging with a solution containing gelatin, sugar and water in a ratio of 2:20:50 . It is better, of course, if you have money, immediately take some industrial gas filtering mask or a protective panoramic mask and kill two birds with one stone (= save on glasses).
The next weak point after the respiratory organs when working with solvents is open skin. If the face is hidden under a gas mask - hands remain. Many solvents are perfectly absorbed through the skin (toluene, tetrahydrofuran) and can cause the strongest dermatitis and eczema (benzene, methylene chloride, chloroform, etc.). Therefore, the best option would be a) the use of protective gloves (gloves made of polyvinyl alcohol - for organochlorine, all others, like latex or nitrile - are suitable only for alcohols, ketones), b) the use of special protective ointments and pastes.
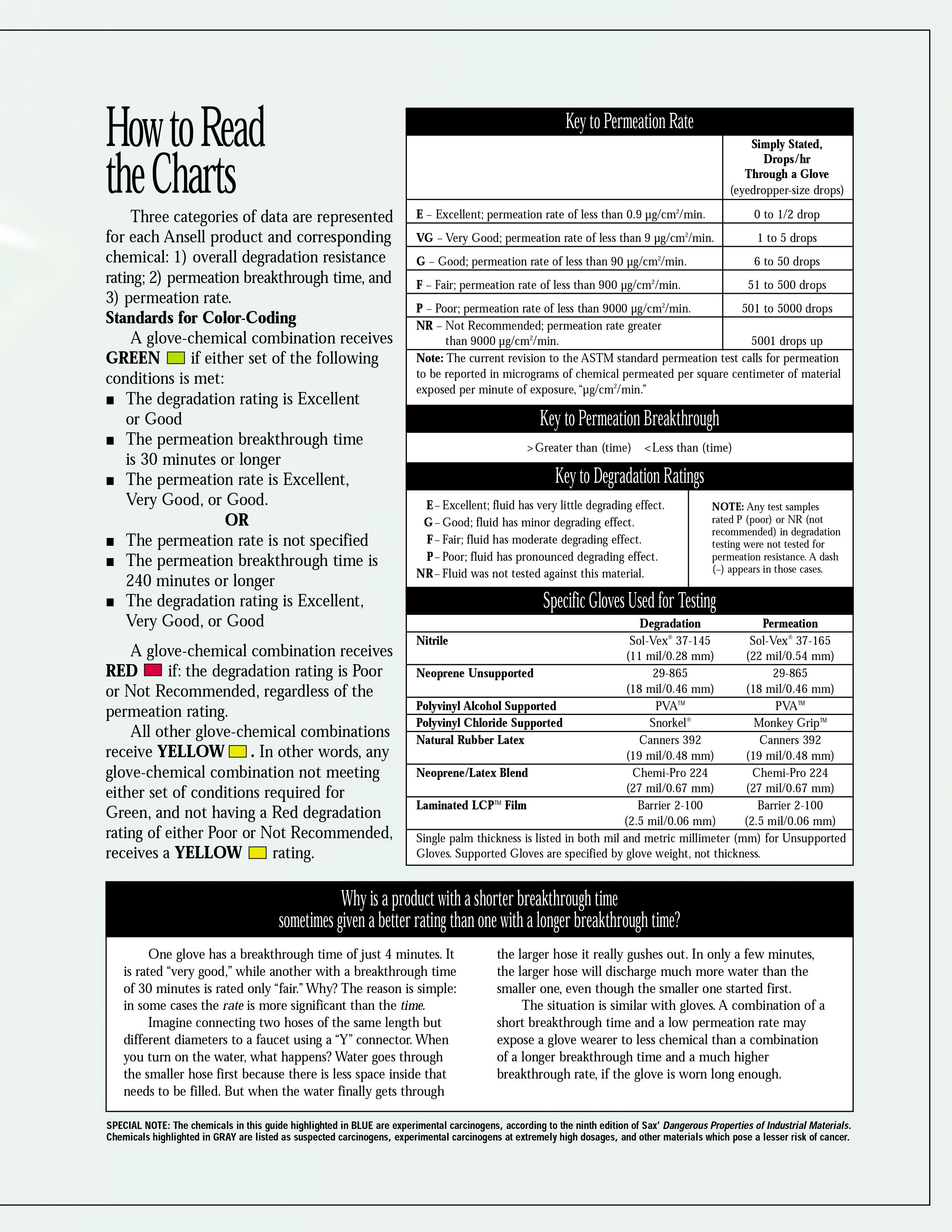
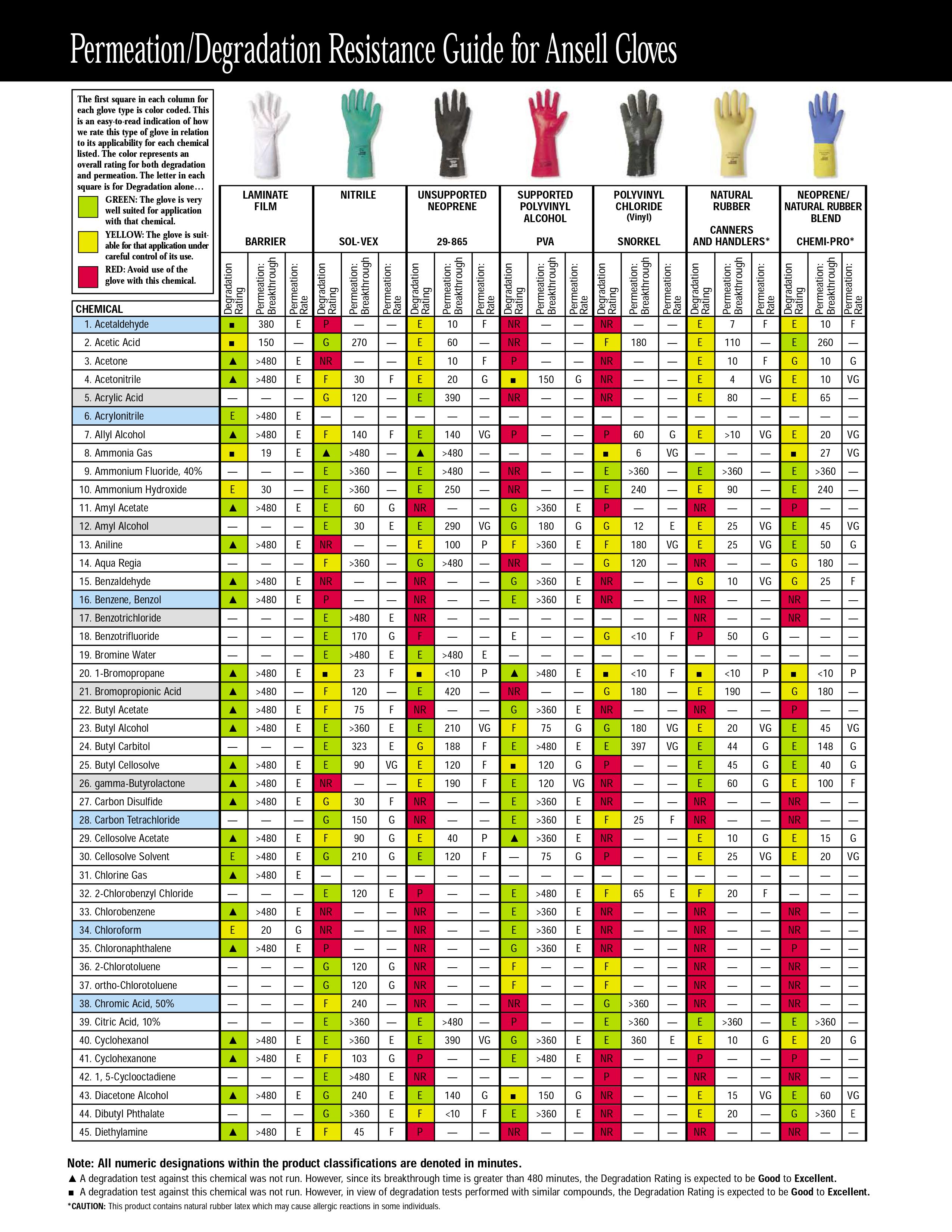
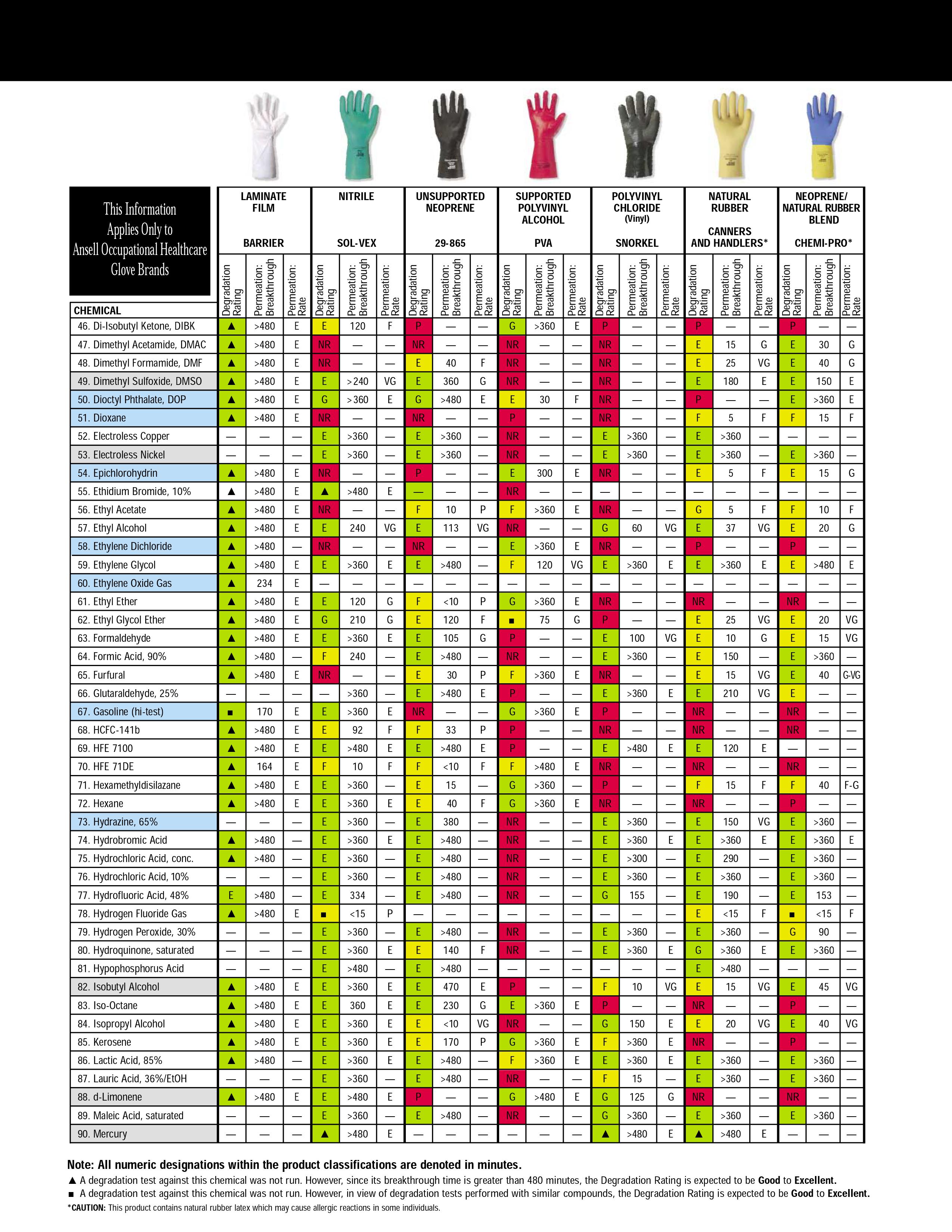
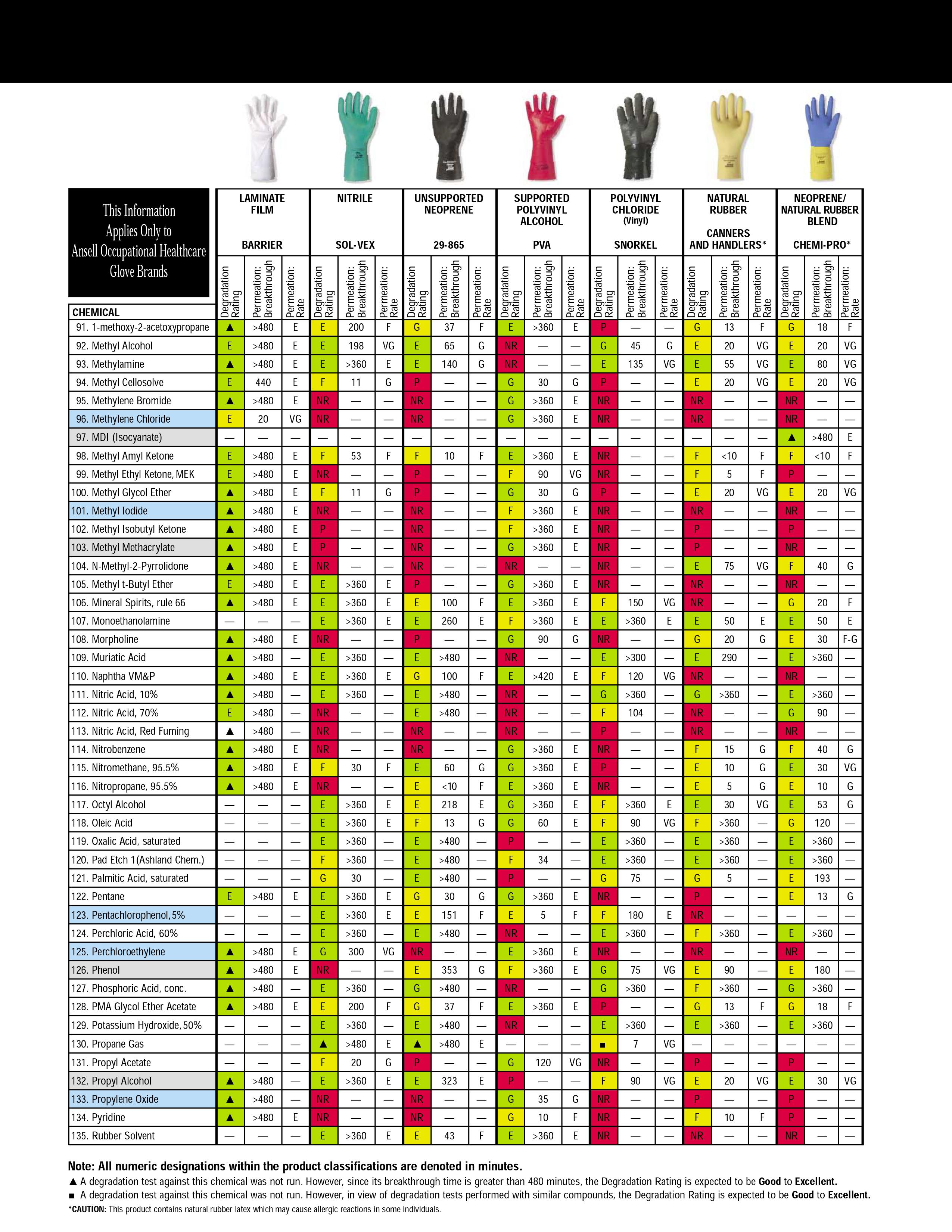
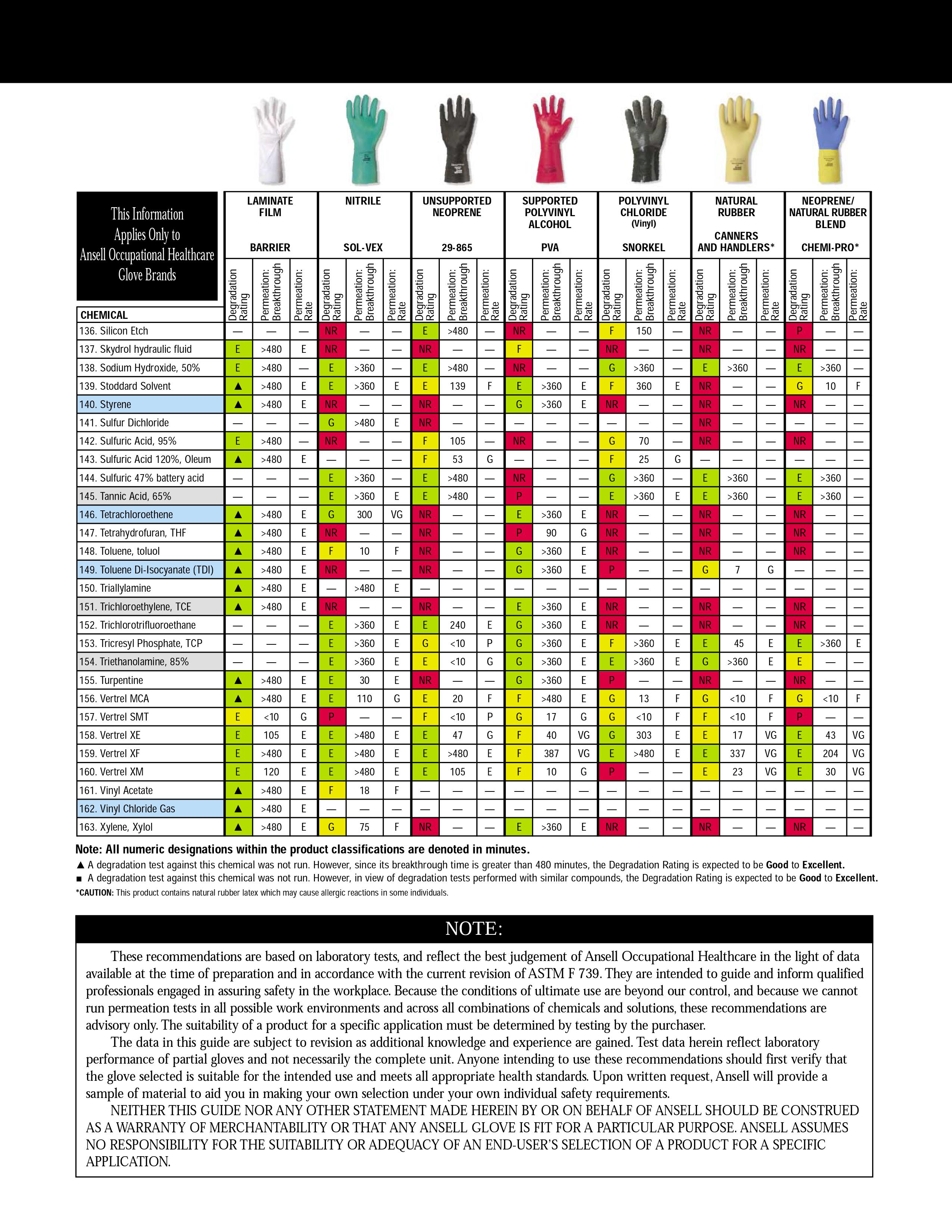
Addition: under the spoiler there are hidden tables of the material resistance of protective gloves to various solvents, found by Kriminalist , for which he thanks a lot. Highly recommended for viewing before purchasing "protective equipment"
When working with aromatic solvents (toluene, benzene, solvents, xylenes), pastes are used: IER-1, HIOT-6, PM-1, YALOT. When working with naphthenic, paraffinic and mixed solvents - YALOT, CHIOT-6, IER-1. The compositions of these time-tested ointments (often referred to as “biological gloves”) are shown in the picture below.
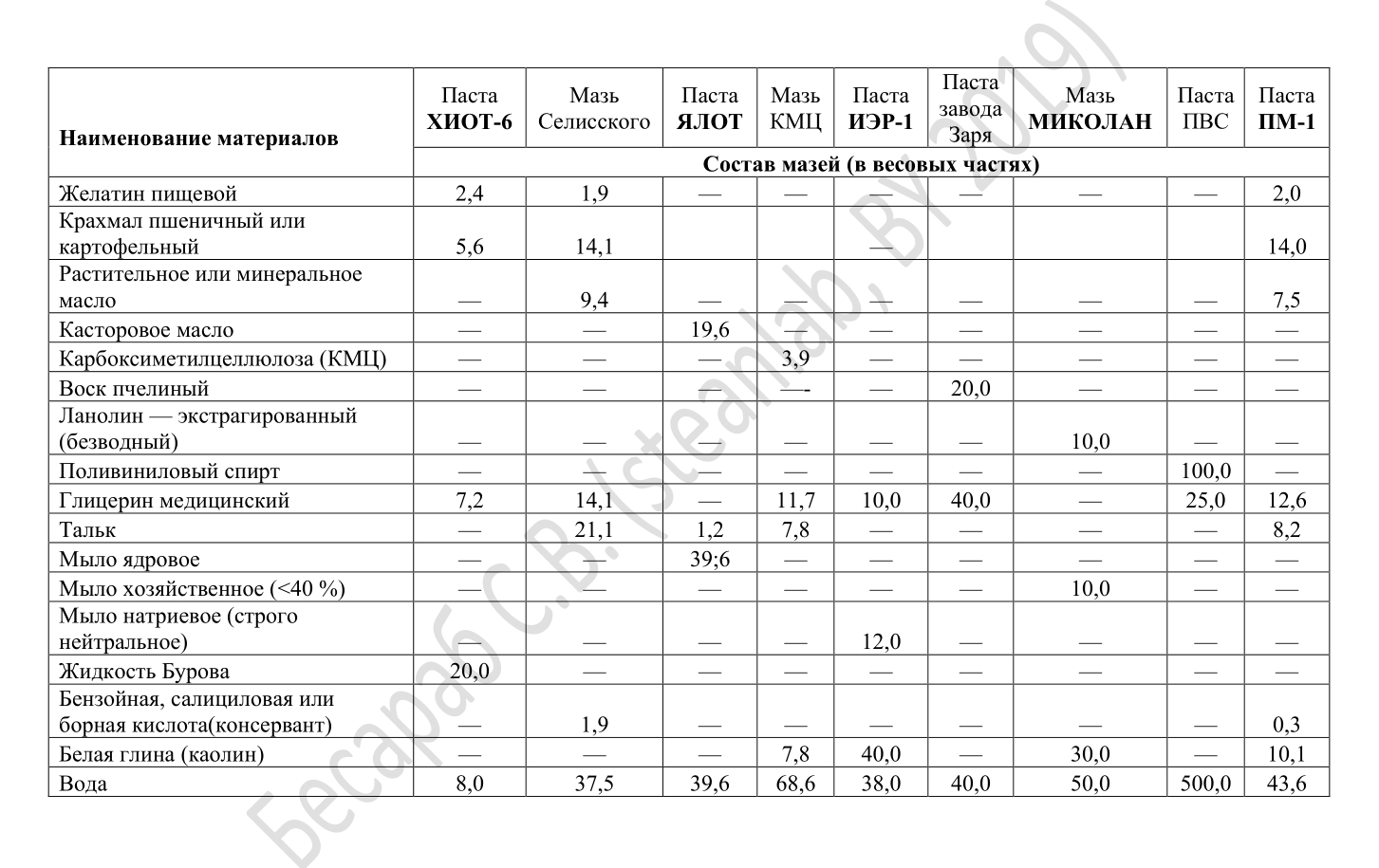
Well, just a few words about clothes. Under normal conditions, there is no such thing as an extraordinary kind of military chemical protection suit. To protect the body is enough workwear (robe) made of cotton fabric. In the case of especially aggressive organochlorine or aromatics, an apron / cape with PVC / PVA or rubber / neoprene coating is added to this.
Note: in Europe there is even a special organization ECSA - E uropean C hlorinated S olvents Association (European Association for Chlorinated Solvents), which annually publishes its bulletins in which it describes in detail the necessary means of protection when working with such solvents, materials, tools, etc.
Summing up, we can say that if the described rules are observed, it will be not only interesting, but also safe to work with solvents. On this otklanivayus, with polymer solutions is finished.
PS Under the spoiler - a table with MPC / description of the physiological effects of common solvents. Taken from the reference book Drinberg S.A. Solvents for paints and varnishes for 1986. So read, but check on the fact of compliance with modern realities (in terms of accuracy of the MPC, it is unlikely that it could increase, but it would decrease completely).
Important!if you did not find your solvent in the table, I strongly recommend using the TOXNET database (Hazardous Substances Data Bank - Database of Hazardous Substances under the auspices of the National Library of Medicine of the USA) and look there.
PPS Appeal to those who ask to check the solubility of a particular plastic in solvents - after the article there is a wonderful “Support the author” button. If a sufficient amount is accumulated - solubility will be possible to check;) Also, these issues can be solved through the consultation system mentioned at the beginning of the article .
Sergey Besarab ( Siarhei V. Besarab )
One of the most frequently asked questions in my consulting practice is issues related to the dissolution / bonding of plastics using various organic solvents. Recently, there has been a real surge of interest in the chemistry of high-molecular compounds, associated with the advent of affordable 3D printers and the need to navigate in the “ink” for them (ie, polymer filaments, filaments). Once again, I am convinced that not even the most advanced “science museum” with a spectacular show can make IT-person more interested in plastics than his own 3D printer. So, reader, if you ever had to think about how to glue plastic, which the default superglue did not glue, if you had doubts about the dissolution of the freshly printed part supports, and it’s just interesting how you can clean the glue from the store price tag on the gift - I ask cat I also strongly recommend that the page be bookmarked not only by those who are often involved in gluing plastics, but also to all those who often have to work with various solvents / thinners. It was done for myself - donated to Habra!

As I wrote a couple of times in the comments to my articles, lately, from time to time I have an idea to make myself an “exhibition” stand, on which samples of plastics would be presented. Just because almost every second chemical question sounds “what kind of plastic is this.” What it says, says that the possibilities of 3D printing have attracted such public attention to plastics, polymers, etc. which hundreds of online popularizers could not have done. Well, in general, looking at these trends, we can safely say that the future, the future is not so much for metals, as for composites and new types of polymers. So, the one who today is thinking about the choice of chemical specialty - consider this option. Therefore, once again, your humble servant decided to make his modest contribution and talk about what I constantly encounter. Today we read about solvents for plastics and the features of working with them. For a start, a little theoretical introduction.
')
“A materiel is that part of the matter ...”
To tell in a few words about the dissolution of polymers will not work with all the desire, because the topic is voluminous and ambiguous (you can even say “pull on a university course”, hello to you, Leonid Petrovich Krul , I pay the debt for 8 on the Navy). A good (read educational) review for people with a fairly high level of technical (chemists and engineers) literacy can be read here . The process of dissolution will be discussed below, in the meantime, a few words about the choice of solvent (or why something dissolves plastic, but something does not).
In general, the selection of a suitable solvent is made by two methods:
1. Using the Hildebrand solubility parameters . This calculation is used if the polymer (p) and solvent (s) have the same polar and hydrogen bond parameter, then the following simple rule works:
| δ s - δ p | ≤ 3.6 MPa 1/2
As an example, I will give the Hildebrand parameters for some polymers:

Who wants to check himself - can calculate solubility at his leisure :). Search for constants can and should be here in this book. It is important to note that the Hildebrand parameters are only useful for non-polar and low-polar mixtures in the absence of hydrogen bonds (dipole moment <2 D (Debye)). For other cases, method 2 is used.
Note: for those who traditionally “knew, but forgot”, I remind you that according to the IUPAC standards (what they look for in the article about the Periodic Table ), solvents are qualitatively grouped into non-polar, polar aprotic and polar proton solvents, for dividing into groups their dielectric constant is often used. Most often, a proton solvent is a solvent that has a hydrogen atom bound to oxygen (as in a hydroxyl group), nitrogen (as in an amino group), or fluorine (as in hydrogen fluoride). In general, any solvent that contains mobile H + is called a proton solvent. Molecules of such solvents easily give protons (H + ) to other reagents. Conversely, protons cannot give aprotic solvents, since they do not contain H + . They usually have high dielectric constant and high polarity. The picture below shows examples of common solvents, divided into classes.

We return to the selection of solvent. As I already wrote, if Hilderbrant did not fit, we use Hansen.
2. Using the Hansen solubility parameters, for each solute one can make an approximate spherical “volume” of solubility with radius R. Only solvents that have Hansen solubility parameters in this volume can dissolve this polymer:
[4 (δ d2 - δ d1 ) 2 + (δ p2 - δ p1 ) 2 + (δ h2 - δ h1 ) 2 ] 1/2 ≤ R
The radius of interaction R depends on the type of polymer. R values are usually in the range of 4 to 15 MPa 1/2 . The parameters of Hansen needed to calculate the solubility of your system can be found in this book . For clarity, the picture below shows the parameters of Hansen (by analogy with Hilderbrant) for some widely used polymers.

If all of a sudden someone really needs to carry out a targeted solvent screening for their polymer using the Hansen method, I recommend paying attention to the HSPiP program, which does an excellent job with this task. Under the link - an overview and description of the work.
In general, we can say the following. First, the “golden rule of dissolution” - like dissolve in like - works for polymers. Those. Compounds with a similar chemical structure are more prone to dissolution than compounds with different structures. Secondly, the higher the molecular weight of the polymer, the closer must be the solubility parameter of the solvent and the polymer for the polymer to dissolve in the solvent. For linear and branched polymers, the graph of solubility versus solubility parameter for a number of solvents reaches a maximum when the solubility parameters (Hansen / Hilderbrandt) of the solute and solvent coincide. In the case of a crosslinked polymer, the volume of swelling, i.e. the absorption of the solvent, will reach a maximum when the solubility parameters of the solvent coincide with those of the polymer. Third, the solubility parameters of polymers do not change much with temperature, while parameters of low molecular weight compounds often decrease markedly with increasing temperature, so the higher the molecular weight of the polymer, the closer the solubility parameter of the solvent must be to dissolve the polymer in the solvent.
Okay, I hope the reader is not tired. I hasten to move from theory to practice.
Chemical welding of plastics
Traditionally, if you suddenly need to grow several pieces of plastic using different methods. Some of them are shown in the picture:

In industry, either welding based on physical methods (such as ultrasonic or laser) or mechanical coupling is often used. Adhesion methods of bonding (glues, melts or polymer solutions) are much less commonly used. Such methods are used in the assembly of plastic windows in stores, gluing together various aquariums, wardrobe trunks and covers. But probably the most popular user of this method is DIY-er, or in our opinion, a homemade man. Ever since the days of the USSR, inventors and simply handy citizens of all stripes glued the hulls of their Plexiglas and dichloroethane articles. With the advent of affordable 3D printers in our life, polymer solutions have received a second life in the form of props, which are created during printing and which need to be somehow removed in the finished product. It is not always possible (and advisable) to do it mechanically, so often his majesty the Solvent of Plastics comes into play.
Note : if you speak for yourself, despite the possibility to print a model on a 3D printer, I still use the old-fashioned glue on plexiglass when I need to make a box or something like that (without Bezier curves). At the KDPV, by the way, an example of such a “momentary!” Is shown. things ", which in haste was glued with a red plexiglass solution (PMMA) from a cone.
So, chemical plastic welding is the process of combining plastic-softened plastic surfaces. The solvent temporarily converts the polymer to a “rarefied” state at room temperature. When this happens, the polymer chains can move freely in the liquid and can be mixed with other similarly dissolved chains. After some time, the solvent due to diffusion and evaporation will penetrate through the polymer and migrate into the environment, and the polymer chains will be compacted (~ packed) and lose their mobility. A frozen ball of tangled polymer chains is a weld with this type of welding. Graphically, the mechanism of the plastic dissolution process is shown in the picture below:

Normally, a normal dissolution includes a solvent penetration stage, a polymer swelling stage, and a polymer diffusion stage into a solvent. The initially glassy polymer contains a multitude of microchannels and holes of molecular size (per so-called infiltration layer).

Upon contact with the solvent, the latter fills these channels and holes and starts the diffusion process (new channels are not formed). Schematically, such a surface layer of a dissolving polymer looks like this (roughly speaking, “glue” = gelatinous mass, which is in the middle between a solid polymer and a liquid solvent):

With the mechanism, I hope, everything is more or less clear, it is time to move on to the specifics of what and what. In the theoretical part, I briefly tried to explain how the solvent screening process takes place for a particular type of polymer. Those. universal and comprehensive table for the dissolution of polymers yet.
And this topic is relevant. Confirmation is the fact that quite often on the pages of various thematic resources (DIY, 3D, amateur radio, etc.) with a noticeable periodicity, there are questions like "how to handle" / "what to glue" / "how to dissolve" this or that type of plastic . It is interesting that in most cases the answers are given by people with the chemistry of polymers (IUDs) who are familiar to me, apparently rather weakly. As a result, there is even more confusion and "space for creativity" of all kinds of amateurs, vendors and other obscurantism. They lose money and time, traditionally, innocent users. So, we look at the table below and we shake on a mustache.

The dark square in the table at the intersection of the "polymer" - "solvent" lines indicates that it is possible to carry out chemical welding using these components. Note : the square at the intersection of "ABS" - "acetone" - with the letter H , because it was the Habra community that convinced me that ABS glues mainly with acetone (I dissolved ABS with acetone, but then I couldn’t glue this solution, for crumbled).
If, as a rule, there are no problems with the issue of plastic, then quite often there is a problem with the presence of the required solvent. Everyone gets to the best of their abilities - someone just orders the necessary solvents, someone searches for them at the flea market, well, and someone is trying to empirically choose from what is sold in stores. Under the spoiler, if that, the composition of commercially available solvents for varnishes and paints (taken from the chemister ).
Where can I get welding electrodes for plastics?
Solvents:
Solvent 645: toluene 50%, butyl acetate 18%, ethyl acetate 12%, butanol 10%, ethanol 10%.
Solvent 646: toluene 50%, ethanol 15%, butyl acetate (or amylacetate) 10%, butanol 10%, ethyl cellosolve 8%, acetone 7%.
Solvent 647: toluene (or pyrobenzene) 41.3%, butyl acetate (or amylacetate) 29.8%, ethyl acetate 21.2%, butanol 7.7%.
Solvent 648: butyl acetate 50%, toluene 20%, butanol 20%, ethanol 10%.
Solvent 649: xylene 50%, ethyl cellosolve 30%, isobutanol 20%.
Solvent 650: xylene 50%, butanol 30%, ethyl cellosolve 20%.
Solvent 651: white spirit 90%, butanol 10%.
Solvent KP-36: butanol 80%, butyl acetate 20%.
Solvent -4: toluene 62%, acetone 26%, butyl acetate 12%.
Solvent R-10: xylene 85%, acetone 15%.
Solvent R-12: toluene 60%, butyl acetate 30%, xylene 10%.
Solvent R-14: cyclohexanone 50%, toluene 50%.
Solvent R-24: solvent 50%, xylene 35%, acetone 15%.
Solvent R-40: toluene 50%, ethyl cellosolve 30%, acetone 20%.
Solvent R-219: toluene 34%, cyclohexanone 33%, acetone 33%.
Solvent -3160: butanol 60%, ethanol 40%.
Solvent RCCH: xylene 90%, butyl acetate 10%.
Solvent RML: ethanol 64%, ethyl cellosolve 16%, toluene 10%, butanol 10%.
Solvent RML-315: toluene 25%, xylene 25%, butyl acetate 18%, ethyl cellosolve 17%, butanol 15%.
Solvent PC-1: toluene 60%, butyl acetate 30%, xylene 10%.
PC-2 solvent: 70% white spirit, 30% xylene.
RFG solvent: ethanol 75%, butanol 25%.
RE-1 solvent: xylene 50%, acetone 20%, butanol 15%, ethanol 15%.
RE-2 solvent: solvent 70%, ethanol 20%, acetone 10%.
RE-3 solvent: solvent 50%, ethanol 20%, acetone 20%, ethyl cellosolve 10%.
RE-4 solvent: solvent 50%, acetone 30%, ethanol 20%.
Solvent FK-1 (?): Absolute alcohol (99.8%) 95%, ethyl acetate 5%
Thinners:
Thinner for water-based paints and varnishes: butanol 62%, butyl cellosolve 38%.
Thinner M: ethanol 65%, butyl acetate 30%, ethyl acetate 5%.
Thinner P-7: cyclohexanone 50%, ethanol 50%.
Thinner P-197: xylene 60%, butyl acetate 20%, ethyl cellosolve 20%.
RDV diluent: toluene 50%, butyl acetate (or amylacetate) 18%, butanol 10%, ethanol 10%, ethyl acetate 9%, acetone 3%.
Thinner RKB-1: xylene 50%, butanol 50%.
Thinner RKB-2: butanol 95%, xylene 5%.
Thinner RKB-3: xylene 90%, butanol 10%.
Electric paint thinners:
RE-1B diluent: solvent 70%, butanol 20%, diacetone alcohol 10%.
RE-2B diluent: solvent 60%, butyl acetate 20%, ethyl cellosolve 20%.
RE-3B diluent: solvent 50%, butanol 30%, ethyl cellosolve 20%.
RE-4B diluent: ethyl cellosolve 50%, solvent 50%.
RE-5B diluent: xylene 40%, cyclohexanone 25%, ethyl cellosolve 25%, butanol 10%.
RE-6B diluent: solvent 50%, xylene 35%, diacetone alcohol 15%.
RE-7B diluent: xylene 60%, butyl acetate 25%, diacetone alcohol 10%, cyclohexanone 5%.
RE-8B thinner: butanol 75%, xylene 25%.
RE-9B diluent: solvent 50%, butyl acetate 30%, ethyl cellosolve 20%.
Thinner RE-10B: solvent 40%, butanol 40%, ethyl cellosolve 20%.
RE-11B diluent: xylene 40%, ethyl cellosolv 30%, butyl acetate 20%, cyclohexanone 10%.
Thinners:
DMZ-R thinner: butyl acetate (or amylacetate) 39%, toluene 30%, ethyl acetate 16%, acetone 15%.
R-5 thinner: xylene 40%, butyl acetate 30%, acetone 30%.
R-6 thinner: pyrobenzene 40%, ethanol 30%, butanol 15%, butylacetate 15%.
P-60 thinner: ethanol 70%, ethyl celloslose 30%.
RVL thinner: chlorobenzene 50%, ethyl celloslose 50%.
Solvent 645: toluene 50%, butyl acetate 18%, ethyl acetate 12%, butanol 10%, ethanol 10%.
Solvent 646: toluene 50%, ethanol 15%, butyl acetate (or amylacetate) 10%, butanol 10%, ethyl cellosolve 8%, acetone 7%.
Solvent 647: toluene (or pyrobenzene) 41.3%, butyl acetate (or amylacetate) 29.8%, ethyl acetate 21.2%, butanol 7.7%.
Solvent 648: butyl acetate 50%, toluene 20%, butanol 20%, ethanol 10%.
Solvent 649: xylene 50%, ethyl cellosolve 30%, isobutanol 20%.
Solvent 650: xylene 50%, butanol 30%, ethyl cellosolve 20%.
Solvent 651: white spirit 90%, butanol 10%.
Solvent KP-36: butanol 80%, butyl acetate 20%.
Solvent -4: toluene 62%, acetone 26%, butyl acetate 12%.
Solvent R-10: xylene 85%, acetone 15%.
Solvent R-12: toluene 60%, butyl acetate 30%, xylene 10%.
Solvent R-14: cyclohexanone 50%, toluene 50%.
Solvent R-24: solvent 50%, xylene 35%, acetone 15%.
Solvent R-40: toluene 50%, ethyl cellosolve 30%, acetone 20%.
Solvent R-219: toluene 34%, cyclohexanone 33%, acetone 33%.
Solvent -3160: butanol 60%, ethanol 40%.
Solvent RCCH: xylene 90%, butyl acetate 10%.
Solvent RML: ethanol 64%, ethyl cellosolve 16%, toluene 10%, butanol 10%.
Solvent RML-315: toluene 25%, xylene 25%, butyl acetate 18%, ethyl cellosolve 17%, butanol 15%.
Solvent PC-1: toluene 60%, butyl acetate 30%, xylene 10%.
PC-2 solvent: 70% white spirit, 30% xylene.
RFG solvent: ethanol 75%, butanol 25%.
RE-1 solvent: xylene 50%, acetone 20%, butanol 15%, ethanol 15%.
RE-2 solvent: solvent 70%, ethanol 20%, acetone 10%.
RE-3 solvent: solvent 50%, ethanol 20%, acetone 20%, ethyl cellosolve 10%.
RE-4 solvent: solvent 50%, acetone 30%, ethanol 20%.
Solvent FK-1 (?): Absolute alcohol (99.8%) 95%, ethyl acetate 5%
Thinners:
Thinner for water-based paints and varnishes: butanol 62%, butyl cellosolve 38%.
Thinner M: ethanol 65%, butyl acetate 30%, ethyl acetate 5%.
Thinner P-7: cyclohexanone 50%, ethanol 50%.
Thinner P-197: xylene 60%, butyl acetate 20%, ethyl cellosolve 20%.
RDV diluent: toluene 50%, butyl acetate (or amylacetate) 18%, butanol 10%, ethanol 10%, ethyl acetate 9%, acetone 3%.
Thinner RKB-1: xylene 50%, butanol 50%.
Thinner RKB-2: butanol 95%, xylene 5%.
Thinner RKB-3: xylene 90%, butanol 10%.
Electric paint thinners:
RE-1B diluent: solvent 70%, butanol 20%, diacetone alcohol 10%.
RE-2B diluent: solvent 60%, butyl acetate 20%, ethyl cellosolve 20%.
RE-3B diluent: solvent 50%, butanol 30%, ethyl cellosolve 20%.
RE-4B diluent: ethyl cellosolve 50%, solvent 50%.
RE-5B diluent: xylene 40%, cyclohexanone 25%, ethyl cellosolve 25%, butanol 10%.
RE-6B diluent: solvent 50%, xylene 35%, diacetone alcohol 15%.
RE-7B diluent: xylene 60%, butyl acetate 25%, diacetone alcohol 10%, cyclohexanone 5%.
RE-8B thinner: butanol 75%, xylene 25%.
RE-9B diluent: solvent 50%, butyl acetate 30%, ethyl cellosolve 20%.
Thinner RE-10B: solvent 40%, butanol 40%, ethyl cellosolve 20%.
RE-11B diluent: xylene 40%, ethyl cellosolv 30%, butyl acetate 20%, cyclohexanone 10%.
Thinners:
DMZ-R thinner: butyl acetate (or amylacetate) 39%, toluene 30%, ethyl acetate 16%, acetone 15%.
R-5 thinner: xylene 40%, butyl acetate 30%, acetone 30%.
R-6 thinner: pyrobenzene 40%, ethanol 30%, butanol 15%, butylacetate 15%.
P-60 thinner: ethanol 70%, ethyl celloslose 30%.
RVL thinner: chlorobenzene 50%, ethyl celloslose 50%.
To the note: I ’ll add a few words about polymers that are not in the table. Of course, this is the favorite "popular" filament - PLA , which dissolves best in polar aprotic solvents : pyridine, N-methylpyrrolidone, ethyl acetate, propylene carbonate, dioxane, dioxane, dichloromethane, chloroform, acetone (?? - depends on the manufacturer of the PLA-filament and contained within “additives”, the same is true for other polymers), nitrobenzene, acetonitrile, dimethylacetamide, etc. A promising 3D polymer PEEK (also known as polyetherketone) is remarkably soluble in 4-chlorophenol (a more rigid variant is a mixture of 80% chloroform and 20% dichloroacetic acid). Chlorophenols (not only 4-, but also 2-chlorophenol) can also dissolve widely distributed and hotly beloved PET . At the request of readers, I will mention a rather new polymer of the PET series, the so-called PETG (polyethylene terephthalate glycol). Like the elder brother, this polymer is resistant to a number of widely available components, it is soluble only in HFIP (hexafluoropropanol). Soft and malleable TPU (thermoplastic polyurethane), like other polyurethanes, can be dissolved in N, N-dimethylformamide (DMF), tetrahydrofuran, ethyl acetate, cyclohexanone, dimethylacetamide. By the way, polyurethane foam, it is also polyurethane. I didn’t see what was in the composition of special liquids for flushing guns for polyurethane foam, but I suspect that some of the mentioned components are definitely there. PCL polymer (polycaprolactone) is dissolved in anisole , 2,2,2-trifluoroethanol, N, N-dimethylformamide, methylpyrrolidone, tetrahydrofuran, dichloromethane, acetone, chloroform and DMSO (dimethyl sulfoxide, which is also sold in the pharmacy "Dimexide"). PDMS (polydimethylsiloxane) widely used for prototyping (especially in scientific institutions related to micro- and nanofluidics) is dissolved using glacial acetic acid. By the way, many other silicones have similar properties, starting from a two-component construction and ending with those on which stickers are glued with prices (therefore, to wash off the adhesive residue from the price tag with ABS plastic, for example, it will be most productive with some acetic acid). Well, in the end a little exotic. EVA (ethylene vinyl acetate), PP (polypropylene), PE (polyethylene, LD / HD) are dissolved in 1,2,4-trichlorobenzene, and PVP (polyvinylpyrrolidone) - in dimethylacetamide.
Safety precautions when working with solvents
Since solvents, to put it mildly, this is not the scent of cherry blossoms for you, there is a safety issue when working with them. It is sad to see how young guys sometimes work without acetone, with chloroform, and even with benzene. And the rules of TB, as you know, are “written in blood” ...
The main ways in which solvents enter the human body (and their vapors) are through the respiratory organs and through the skin. I don’t consider any deviations (like ingestion), because a person in their right mind will never drink benzene. The above reagents have a predominantly narcotic effect, have a pronounced irritant effect on the mucous membranes of the upper respiratory tract and conjunctiva of the eye, moderate - on the skin. The best protection against them is to work in the conditions of supply and exhaust ventilation, in special boxes. If it happens in specialized workshops or laboratories, then most often there is already a fume hood .

If it is impossible to arrange the necessary ventilation, working with organic solvents provide personal protective equipment: respirators, gas masks, oxygen-insulating devices, etc. (depending on the vapor concentration). In general, solvent vapors are remarkably sorbed by activated carbon (and many other sorbents), not without reason, some solvents were actively used to assess the sorption capacity of a material (the so-called “exicator method”). I personally “had the honor” to check the sorption capacity of coals by their absorption of carbon tetrachloride CCl 4 . Most of the vapor can hold a gas mask with a class A box or a respirator mask with a similar filter cartridge. Like this:

It is important in the description to look for something like " protects against vapors of organic compounds (gasoline, kerosene, acetone, benzene and its homologues, xylene, carbon disulfide, etc.), phosphorus and organochlorine toxic chemicals, dust, smoke, fog ." But to such a mask, it is also desirable to have sealed glasses, whose glasses are rubbed with fogging with a solution containing gelatin, sugar and water in a ratio of 2:20:50 . It is better, of course, if you have money, immediately take some industrial gas filtering mask or a protective panoramic mask and kill two birds with one stone (= save on glasses).
My favorite protective equip (after traction)
Mentioned already panoramic mask (excellent visibility after a gas mask from the USSR)

But she, on the other hand

And my pride filtering box with mercury vapor protection.


But she, on the other hand

And my pride filtering box with mercury vapor protection.

The next weak point after the respiratory organs when working with solvents is open skin. If the face is hidden under a gas mask - hands remain. Many solvents are perfectly absorbed through the skin (toluene, tetrahydrofuran) and can cause the strongest dermatitis and eczema (benzene, methylene chloride, chloroform, etc.). Therefore, the best option would be a) the use of protective gloves (gloves made of polyvinyl alcohol - for organochlorine, all others, like latex or nitrile - are suitable only for alcohols, ketones), b) the use of special protective ointments and pastes.
Addition: under the spoiler there are hidden tables of the material resistance of protective gloves to various solvents, found by Kriminalist , for which he thanks a lot. Highly recommended for viewing before purchasing "protective equipment"
When working with aromatic solvents (toluene, benzene, solvents, xylenes), pastes are used: IER-1, HIOT-6, PM-1, YALOT. When working with naphthenic, paraffinic and mixed solvents - YALOT, CHIOT-6, IER-1. The compositions of these time-tested ointments (often referred to as “biological gloves”) are shown in the picture below.

Well, just a few words about clothes. Under normal conditions, there is no such thing as an extraordinary kind of military chemical protection suit. To protect the body is enough workwear (robe) made of cotton fabric. In the case of especially aggressive organochlorine or aromatics, an apron / cape with PVC / PVA or rubber / neoprene coating is added to this.
Note: in Europe there is even a special organization ECSA - E uropean C hlorinated S olvents Association (European Association for Chlorinated Solvents), which annually publishes its bulletins in which it describes in detail the necessary means of protection when working with such solvents, materials, tools, etc.
Summing up, we can say that if the described rules are observed, it will be not only interesting, but also safe to work with solvents. On this otklanivayus, with polymer solutions is finished.
PS Under the spoiler - a table with MPC / description of the physiological effects of common solvents. Taken from the reference book Drinberg S.A. Solvents for paints and varnishes for 1986. So read, but check on the fact of compliance with modern realities (in terms of accuracy of the MPC, it is unlikely that it could increase, but it would decrease completely).
Important!if you did not find your solvent in the table, I strongly recommend using the TOXNET database (Hazardous Substances Data Bank - Database of Hazardous Substances under the auspices of the National Library of Medicine of the USA) and look there.
Solvents. MPC & body effects

PPS Appeal to those who ask to check the solubility of a particular plastic in solvents - after the article there is a wonderful “Support the author” button. If a sufficient amount is accumulated - solubility will be possible to check;) Also, these issues can be solved through the consultation system mentioned at the beginning of the article .
Sergey Besarab ( Siarhei V. Besarab )
Used sources
.. : . .: , 1986.
.. . ., , 1989.
. . . , ., 1954.
Yue CY. The structure and strength of solvent welds between dissimilar amorphous thermoplastics. International Journal of Adhesion and Adhesives, 8(1), p. 47, 1988.
Tres P: Assembly techniques for plastics. Designing Plastic Parts for Assembly, Reference book (ISBN 1-569-90199-6), Hanser Gardner Publications, Inc., 1995.
Rosato's Plastics Encyclopedia and Dictionary, Reference book (ISBN 3-446-16490-1), Carl Hanser Verlag, 1993.
Desai J, Barry CMF, Mead JL, Staceer RG: Solvent welding of ABS and HIPS: a case study in methylene chloride substitution. ANTEC 2001, Conference proceedings, Society of Plastics Engineers, Dallas, May 2001.
Warwick CM Solvent welding. Handbook of Adhesion, 2nd Edition, Reference book (ISBN 0-471-80874-1), John Wiley & Sons, 2005.
Lowery TH Mechanism and Theory in Organic Chemistry, Harper Collins Publishers 3rd ed. 1987
Sato, S., Gondo, D., Wada, T., Kanehashi, S., & Nagai, K. (2012). Effects of various liquid organic solvents on solvent-induced crystallization of amorphous poly(lactic acid) film. Journal of Applied Polymer Science, 129(3), 1607–1617.
Grewell, D. Plastic and Composite Welding Handbook, Hanser Publishers, Munich (2003)
Xu, J., Zhang, Z., Xiong, X., & Zeng, H. (1992). A new solvent for poly(ether ether ketone). Polymer, 33(20), 4432–4434.
AFM Barton, CRC Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters, CRC Press, Boca Raton, 1991.
Charles M. Hansen, Hansen Solubility Parameters: A User's Handbook, 2nd Edition, 2007
Beth A. Miller-chou, Jack L. Koenig A review of polymer dissolution. Prog. Polym. Sci. 2003
.. . ., , 1989.
. . . , ., 1954.
Yue CY. The structure and strength of solvent welds between dissimilar amorphous thermoplastics. International Journal of Adhesion and Adhesives, 8(1), p. 47, 1988.
Tres P: Assembly techniques for plastics. Designing Plastic Parts for Assembly, Reference book (ISBN 1-569-90199-6), Hanser Gardner Publications, Inc., 1995.
Rosato's Plastics Encyclopedia and Dictionary, Reference book (ISBN 3-446-16490-1), Carl Hanser Verlag, 1993.
Desai J, Barry CMF, Mead JL, Staceer RG: Solvent welding of ABS and HIPS: a case study in methylene chloride substitution. ANTEC 2001, Conference proceedings, Society of Plastics Engineers, Dallas, May 2001.
Warwick CM Solvent welding. Handbook of Adhesion, 2nd Edition, Reference book (ISBN 0-471-80874-1), John Wiley & Sons, 2005.
Lowery TH Mechanism and Theory in Organic Chemistry, Harper Collins Publishers 3rd ed. 1987
Sato, S., Gondo, D., Wada, T., Kanehashi, S., & Nagai, K. (2012). Effects of various liquid organic solvents on solvent-induced crystallization of amorphous poly(lactic acid) film. Journal of Applied Polymer Science, 129(3), 1607–1617.
Grewell, D. Plastic and Composite Welding Handbook, Hanser Publishers, Munich (2003)
Xu, J., Zhang, Z., Xiong, X., & Zeng, H. (1992). A new solvent for poly(ether ether ketone). Polymer, 33(20), 4432–4434.
AFM Barton, CRC Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters, CRC Press, Boca Raton, 1991.
Charles M. Hansen, Hansen Solubility Parameters: A User's Handbook, 2nd Edition, 2007
Beth A. Miller-chou, Jack L. Koenig A review of polymer dissolution. Prog. Polym. Sci. 2003
Source: https://habr.com/ru/post/447794/
All Articles