Radiation: an invisible killer and his daughters or a little about radon

In previous articles and discussing them, I have repeatedly stated: no modern methods can reliably detect the effects of the magnitude of natural background radiation in a sufficiently wide range of it on people's health. But there is one natural radiation factor, the effect of which is relatively well marked. This is a radioactive inert gas radon , nicknamed by journalists as an "invisible killer."
Radium emanation
In 1899, Rutherford and Owens found that, in addition to radioactive radiation, thorium secretes a certain substance, which also possesses the basic property of radioactive radiation — the ability to ionize — behaves like a gas: it is transported with an air flow, but does not spread in a straight line, diffuses through porous media, lingering in the thinnest continuous partitions, and in addition, it “settles” on objects placed in its surroundings, informing them of radioactivity that is rapidly decreasing according to the exponential law. This was unusual: before that, radioactivity seemed to be an exceptionally constant phenomenon. At the same time with them and not knowing anything about their work, a similar phenomenon was observed by the German Friedrich Dorn, who worked with radium and also released radioactive gas from it. The gas released from radioactive substances was called emanation. The emanations of radium and thorium turned out to be unequal and above all, had a different half-life: 3.8 days for radium and 55 seconds for thorium.
Rutherford and Soddy, who joined him, studied the nature of the emanations. In the spectrum of the gas discharge in the emanation were present lines of helium. Moreover, their intensity rapidly increased simultaneously with a drop in the intensity of radiation from the tube with emanation. The connection of helium with radioactive minerals was already known: on earth, for the first time, it was isolated from thorium-containing minerals. When in 1903 it was possible to collect a sufficient amount of emanation, it was possible to see the spectrum of the emanation itself, which differed from the spectra of all other gases. It was not a spectrum of helium: it was a spectrum of a new chemical element.
Emanation was not helium. But she turned into him! Its spectrum weakened over time, and in its place appeared the familiar spectrum of helium with its yellow line next to the sodium doublet. It was something new and incredible: scientists watched as one chemical element turned into another in their eyes.
')
The most difficult task fell to the role of William Ramzai: he managed to isolate a tiny amount of new gas in a free form and he managed to determine its density. The molecular mass calculated from it turned out to be 222, which was exactly four times less than the atomic mass of radium — the atomic mass of helium.
It turned out that radium turned into helium and emanation. And then the emanation turned into helium - and something else.
Further studies of Rutherford identified the alpha particles with the helium atoms, and the picture was finally formed. The fact of the existence of a fundamentally new natural phenomenon — the transformation of some elements into others with the emission of rapidly flying particles — was reliably established. And it broke all the scientific ideas that barely had time to emerge. Not so long ago, the concept of an atom — the elemental indivisible and unchanging unit of matter — was formed, as it turned out that an atom can suddenly disintegrate, and its two new atoms of other chemical elements will be its “fragments”.
In the meantime, the emanation by the efforts of Ramsay took its place in the periodic system, adding another family of inert gases to another element and was later renamed radon.
Radon as a substance
From a chemical point of view, radon is an inert gas. Like xenon, it is not as inert as helium, neon, or argon, and, unlike the latter, has some chemical properties . However, in ordinary life, they can be safely neglected: the ability of radon to enter into chemical compounds is too small. But it is easily adsorbed by tissues, paper, activated carbon and silica gel, dissolves in oils and actively goes into ice from solution in water when it freezes, forming clathrates. Also, radon forms stable clathrates with a number of other molecular compounds — thus, radon with glucose is well known and used in “radon medicine”.
Pure radon glows due to radioactivity. Especially bright blue light - liquid radon glows, which upon further cooling freezes and when approaching the temperature of liquid nitrogen changes the color of the glow to yellow, and then to orange. With the accumulation of decay products, liquid and solid radon, initially colorless, darkens.
But outside of special laboratories and hot cells, we will never see either liquid or solid radon. Even gaseous, it is found in nature only in very small concentrations. After all, a gram of radium per day forms only radon Therefore, the only sign of its presence will almost always be only radioactivity - of him and his daughter decay products.
Radon as a radionuclide
A total of 19 radon isotopes are known, but only two radon isotopes can be encountered in ordinary life: radon itself (radium emanation) with an atomic mass of 222 and a short-lived toron with a half-life of 55 seconds and a mass number of 220. There is a third natural radon isotope, actinon , a short-lived a member of the uranium-235-actin series, but due to the short half-life and low content of uranium-235 and its “daughters” in nature, it is difficult to detect. Radon-222, emitting an alpha particle with an energy of 5.59 MeV, turns into polonium-218 (often referred to as the old, still the time of the Curie couple, denoted as RaA) with a half-life of just 3.1 minutes, and the “spitting out” of the alpha again -particle, turns into lead-214 (RaB), or undergoes beta decay, becoming astatine-218 and almost immediately - through alpha decay - bismuth-214 (RaC). Lead-214 turns into the latter. Lead and bismuth-214 half-lives - a little less than half an hour and their atoms, formed after the collapse, have time to condense during this time, forming the so-called active plaque covering the surfaces of dust particles and other aerosol particles. Beta activity makes these motes positively charged. Bismuth-214, emitting beta and alpha particles almost simultaneously (through polonium-214), turns into a rather long-lived (22 years) lead-210, on which the fast chain of transformations stops. The alpha decays of polonium-218 and polonium-214 give the bulk of the dose of internal exposure caused by radon-222. But the dose from radon itself does not exceed 2% of the total dose.
This chain of radionuclides quickly turning into each other - polonium-218, lead-214, bismuth-214, polonium-214, lead-210 - is called the radon decay daughter (DPR) of radon and inseparably accompanies it in the air. Together with radon, we inhale them into our lungs, and when it rains, it washes them out of the air, which is why rainwater acquires radioactivity with a half-life of about 25 minutes. This radioactivity can be easily detected by rubbing any surface in the rain with a cloth and measuring the cloth with a household dosimeter, preferably with a mica sensor (the lead cap on the sensor needs to be removed). At the same time, many take the shocking testimony of the dosimeter as the consequences of the Chernobyl disaster, Fukushima or signs of some kind of accident that the authorities hide , but in fact the reason for this is radon. An increase in the radiation background during heavy rains (and partly with the scattering of cosmic muons on raindrops with the formation of secondary electrons and bremsstrahlung gamma radiation) is partially connected with it.
Toron also lives less than a minute and usually breaks up almost in the same place where it was formed. Having emitted two alpha particles in a row (through living polonium-216 - thorium-A in a fraction of a second), it turns into lead-212 (thorium-B), which lives 10 hours and forms the active pathogen of toron with its "heir" bismuth-212 ( thorium-C) with a half-life of 1 hour. The latter makes a “fork”: in one of its branches, emitting an alpha particle, it turns into thallium-208, famous for its extreme right on the energy scale with a gamma line of 2.6 MeV, and in the other - through beta decay, it turns into polonium-212, which instantly (in microseconds) emits an alpha particle of very high energy (10.5 MeV). In both cases, a stable lead-208 is formed. Because of the small time of life, the toron practically does not have time to scatter and we do not breathe. The radiation hazard is represented by dust-like 212th isotopes, which become a source of alpha-beta and gamma radiation of extremely high energy.
As a characteristic of the content of radon in the air, a quantity commonly called the equivalent equilibrium volume activity (EER) is usually used. It is calculated for radon-222 using the formula:
Where and - the volumetric activity of radon and its daughter decay products (Po-218, Pb-214, Bi-214) in .
Similarly, according to the formula
determine the eroa of radon-220. Here, ThB and ThC are lead and bismuth-212, respectively.
Here - the equilibrium factor, which at full equilibrium is equal to one, but in practice usually does not exceed 0.5.
In the future, speaking of "concentration", "level", "content", etc., I mean it is EERO.
Radon-killer (and a little doctor)
The decay of radon-222 and its daughter products is responsible for about half the dose of natural human exposure. As practically the only natural radionuclide present in the environment in the form of gas (not counting trace amounts of tritium and radiocarbon), radon almost completely forms the dose of radiation from the inside of the lungs. The lungs are an organ of relatively high radiosensitivity due to the constantly renewed alveolar epithelium, therefore the risk of lung cancer when irradiated is about three times higher than the overall risk of oncology with uniform irradiation of the body. And after the decay of radon, its DPR (and later - polonium-210, formed from lead-210 remaining in the lungs, which has the ability to accumulate in the lungs) are fixed in the lung tissue, and irradiated with alpha particles, each of which 6, and a toron - up to 10 MeV, and a quality factor of 20, is a very destructive projectile. For each atom of radon of such "projectiles" there are four, and for an atom of toron - three.
Because of this (and also due to the fact that lung cancer in non-smokers is quite rare), even relatively low levels of radon concentrations affect the incidence of lung cancer. According to the US Public Health Service, radon is the second after smoking cause of the incidence of tumors of this localization. With a radon concentration of 200 The additional risk of lung cancer is 220 cases per year per 1 million people and increases linearly with increasing radon content. For comparison, the risk of lung cancer for non-smokers and smokers is 34 and 590 cases per year per 1 million people (figures taken from lectures by Professor I.N. Beckman).
There is also an opinion that radon, in addition to well-known stochastic effects, also provokes cardiovascular diseases. However, this opinion is usually expressed in connection with an attempt to explain the so-called geopathic zones, the existence of which in itself is rather dubious.
In general, it is radon that is currently the most important problem of protecting the population from radioactive threats. This applies particularly to some regions where radon is actively released from the bowels of the Earth and its concentration in the basements and on the first floors of buildings is extremely high.
Such a place on Earth, for example, is Caucasian Mineral Waters, Beshtau. To assess how serious it is, I recommend watching this video:
Can you imagine what will happen to the lungs of someone who goes there without respiratory protection?
The same situation as in the Caucasian Mineral Waters is observed in other regions known for its granite massifs, volcanoes, hot springs and uranium ores - Switzerland, Austria, the Czech Republic, on a smaller scale - Finland and northwest Russia, as well as southern Siberia, Far East. In these regions, the urgent need is to reduce the concentration of radon in residential premises - radon protection.
The map below shows the doses received from radon by residents of various regions of Russia (in mSv / year).
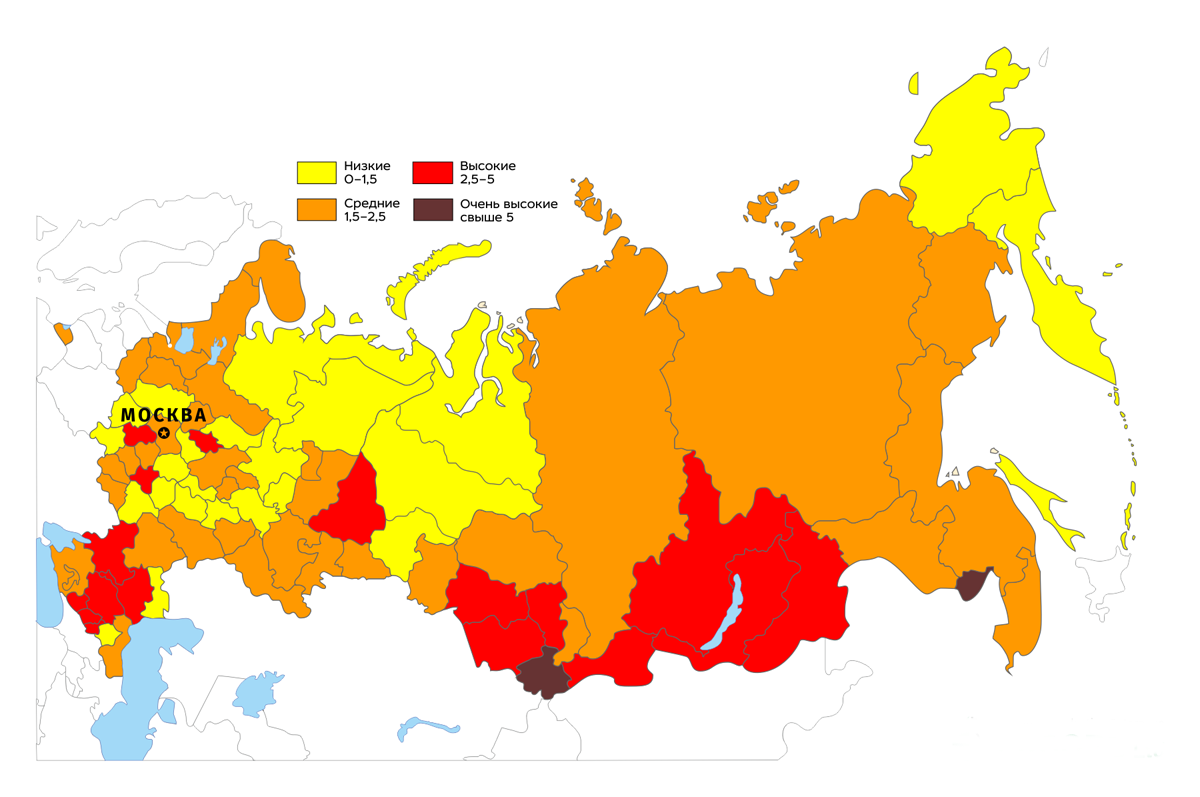
There is, however, the opinion that the radon problem is exaggerated. The above figures for cancer incidence are not experimentally established, but calculated, based on data on the incidence of people living and working at significant levels of radon - miners, workers and residents of radon resorts, etc. At the same time, the non-threshold concept, on the basis of which these figures were calculated, has not been proven experimentally and remains a hypothesis, even if it is well-founded theoretically. As an argument, usually indicate the well-known therapeutic effect of radon in various diseases. It is known that radon has an analgesic and anti-inflammatory effect, causing (probably, by increasing the production of DOPA and related biologically active compounds by skin melanocytes) activation of a number of neuroendocrine mechanisms, which have a pronounced effect on the cardiovascular and nervous systems, as well as enhances microcirculation in the irradiated skin. Radon baths have shown their effectiveness in many diseases.
In addition, there is evidence that the alpha radiation of particles coated with "active plaque" stimulates the activity of pulmonary cilia, helping to remove these particles from the lungs, and this mechanism can significantly reduce the effects of small radon concentrations.
Despite the fact that thorium (by activity) is not less than uranium, the proportion of thoron in the total dose is only about 5%. This is due to the fact that it "does not live" to our lungs, in most cases, just not having time to reach the surface.
Radon sources
The half-life of radon-222 is only 3.8 days, but thanks to its constant formation during the decay of radium, new radon is constantly entering the atmosphere. Sources of radon, therefore, are rocks rich in uranium, mainly granites, but there are also much more active and rich in uranium rocks. So, phosphorites are known for their uranium-bearing nature. But the greatest amount of radon is allocated not from a monolithic granite massif, but from faults leading into the bowels of the Earth, forming the so-called “radon respiration”. The selection of radon is a kind of marker by which such faults can be found, and hence the deposits of various mineral resources associated with them. Radon is particularly intense in volcanic areas. Sometimes they detect an intense release of radon in places where, seemingly, there is no place to go. A detailed study reveals a deep rift. And the intensity of radon release is rich and, most importantly, a fairly fast-acting source of information about changes in the state of the earth's interior. Its fluctuations foreshadow earthquakes and volcanic eruptions, make it possible to predict rock bursts in mines, help prevent accidents during drilling.
Radon is also distinguished from building materials. The “leader” here is phosphogypsum - a material obtained as waste in the production of phosphate fertilizers, which concentrates a large part of the radium phosphate in the initial phosphate (in which, like uranium, it is abundant), so radon phosphogypsum emits a lot. And since the utilization of phosphogypsum is a real problem, the temptation to use it as gypsum in building mixtures is very high. So there are "glowing" and emitting radon drywall plates, self-leveling floors and plaster.
I have already spoken about the radioactivity and "radonogenicity" of granite - and granite gravel and sand often become a component of concrete used in construction. At the same time, it is necessary to be guided by NRB-99 and to use various types of granite of radioactivity where it is permissible. Granite can be divided into 4 classes of radioactivity:
I - up to 370 Bq / kg - is allowed to apply without restrictions in any construction,
II - up to 740 Bq / kg - can be used in non-residential buildings (including public) and for exterior cladding,
III - up to 2800 Bq / kg - only for road construction outside populated areas,
IV - up to 3700 Bq / kg - can be used in construction only where it will be covered with a thick layer of low-active material.
With an activity of more than 3,700 Bq / kg, granite is not used in construction.
At the same time for the preparation of concrete for residential buildings is used only the most low-active granite class I of radioactivity.
The source of radon in the premises can also serve as a mixture of uranium ceramic tile, granite cladding. But usually these sources can be neglected. By the way, uranium glass, which some Russian celebrities (and not only they) so fond of collecting, is not a source of radon danger: radon is not only not able to go beyond the continuous mass of glass, but practically does not form in this glass, since very little radium. When uranium was extracted from the ore, the radium contained in it was removed, and the new one did not have time to form. But samples of uranium minerals and devices with a constant-light light composition based on radium-226 can "flatten" an apartment to quite dangerous levels.
In radon-hazardous regions, the strongest source of radon is water supply, if water for it is taken from artesian wells. So, while taking a shower, the concentration of radon in the room can rise from 50-100 Bq / m ^ 3 to several kilobakerles per cubic meter. Gas also supplies radon to our apartments.
Radon danger dramatically aggravates ... energy saving. It makes it much less airtight at home than before, to air less and less, to actively use air recirculation, which means - radon that has got into the room remains in it. Therefore, the materials and approaches to construction, which in our country lead to acceptable levels of radon, as it increases the fight against heat leakage can give a serious increase.
Detection and measurement
How to find out what the radon level is where you live or work? Unfortunately, this is not very easy. Although radon is the source of half of the natural background radiation, the “normal” readings of the dosimeter are not at all a sign of well-being. In general, radon can be detected by the dosimeter in rare cases of very large levels - with its characteristic features are smooth, wavy fluctuations in dose rate and a rapid decrease in radiation levels when opening doors and windows.
There are a number of “standard” used for official measurements, methods for quantitative determination of radon content. The first of these is the direct counting of alpha decays in the ionization chamber filled with the air under study. Decays are recorded by very weak current pulses, which occur when the charges formed during the passage of alpha particles, or by ionization current, which is usually not measured directly due to its extremely small size, but determine the discharge time of the constructive capacity of the ionization chamber. Another method is scintillation - as a scintillator use a layer of zinc sulfide deposited on the hemispherical inner surface of the working volume, and the “plug” covering the detector is a photomultiplier. Alpha-radiation semiconductor sensors are used in a similar way, but due to the short mileage, it is impossible to make a detector for a large volume of gas, and the measurement time of ordinary radon activities (tens of Bq / m ^ 3) stretches for many hours or even days. The measurement time can be significantly reduced by collecting radon DPRs on the detector surface electrostatically: such well-known instruments as SIRAD MR106N, Radex MR107 work. These are inexpensive devices, the cost of which is comparable to the price of simple dosimeters (about 10,000 rubles). Unfortunately, such devices on the detector eventually accumulate long-lived decay products (lead and polonium-210), gradually increasing the instrumental background, especially when using such devices in areas heavily infected with radon, which requires replacement.
A filtering method is also used. Several cubic meters of air are pumped through a layer of sorbent and then the radioactivity of the sorbent is measured. To do this, use a gamma spectrometer, recording the peaks of lead and bismuth-214. There are specialized devices that include a detector with a gamma-spectrometer and a pump with a filter cell, housed in a single package. These are expensive devices that allow determining the minimum radon activities in a short time and tracking small fluctuations of the radon EED.
The simplest version of this method is not difficult to detect the presence of radon in the apartment - it is enough to use a vacuum cleaner and a Petryanov filter (any respirator), and then measure the filter with a dosimeter with a mica sensor. But in order to measure it quantitatively , it is necessary to standardize the method and calibrate it. And this is almost inaccessible at home. But if the dosimeter showed a much larger than the natural background after a few minutes of the operation of the vacuum cleaner, the value is an occasion to sound the alarm.
The same applies to the well-known “radon trap” method. The trap itself is simple to manufacture: it consists of a voltage multiplier with an output voltage of minus 600-1500 V and a metal plate or grid to which this potential is applied. The multiplier circuit, given by the notorious Oleg Aizon, looks like this:
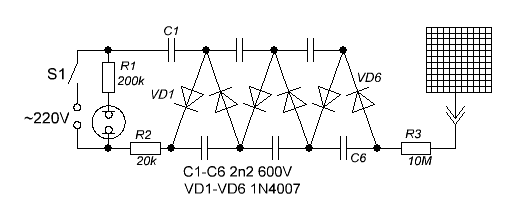
(scheme taken from the forum RCBZ , ibid - almost all of its manufacture and use). The electrode is placed under the negative potential of the measured room and left there for 6-8 hours, and then measured with a radiometer with the gamma filter cover open.
The mechanism of operation of the radon trap is due to the fact that aerosol particles coated with the active deposit of radon DPR, due to beta activity, acquire a positive charge and are attracted to the negatively charged electrode. After some time, between the deposition of new DPR of radon and the decay of those already settled, an equilibrium is established in which the activity of the deposited DPR is proportional to the concentration of radon.
Oleg Aizon cites the following "reference points of the scale":
10-60 μR / h - the normal level of radon,Of course, these figures will significantly depend on what is being measured: the Stora-TU radiometer used by Aison on the SBM-20 counters will give less readings than the radiometer with a mica sensor, for example, MKS-03SA.
70-150 μR / h - increased radon level
150 μR / h and more - there is a source of radon in the room
400-600 μR / h - very high radon content
Of the other "professional" methods for determining radon, track detectors should be noted. The detector itself is very cheap - it is a polycarbonate film, covered with a layer of filter material that does not allow radon and other radioactive dust to pass through to the film, but does not delay radon itself. The film is left for a certain time in the investigated room, mine or well, and then "manifested" by etching. The areas destroyed by alpha particles dissolve in the etchant and there are holes on the film, the number of which is proportional to the radon concentration multiplied by the exposure time. In some countries, such detectors are distributed to residents of radonophore regions with instructions and instructions to send to a specific address after exposure.
* * *
Contrary to popular belief that “everything natural cannot be harmful”, radon may be the cause of death for more people than smoking, car accidents and domestic accidents. So protection from it in radon-prone regions is urgently needed. Whether radon is a harmful factor at relatively low levels is an open question.
Source: https://habr.com/ru/post/445832/
All Articles