Notes phytochemist. Radio banana
Every miracle must find its explanation, otherwise it is simply unbearable ...
K.Chapek
I practically do not touch in my articles of things that are universally described and easily accessible, for example, the macro-and microelement composition of fruits / vegetables. But for a banana decided to make an exception. Banana has a lot of potassium! Lift anyone in the middle of the night and ask what is useful in a banana - you will get the answer “potassium for the heart” (I exaggerate, but not far from the truth). And potassium, it is an element difficult, "with a hint of radioactive ...". In general, to find out if radioactivity from a banana is so great and if it is so terrible, go under the cat.
ps note "at the request of ..."
Potassium refers to the so-called. nutrients , i.e. it is constantly present in a living organism and plays an important biological role. The human body contains about 0.35% potassium. 98% of this amount is in the cells, and the remaining 2% is extracellular fluid (including blood). Gradient concentrations supported by the so-called. "Na + / K + pump". The presence of an electrochemical potassium gradient between the intracellular and extracellular spaces is important for the functioning of the nervous function (repolarization of the cell membrane, for example). When hypokalemia (potassium deficiency) due to slower ventricular repolarization increases the risk of heart rhythm disturbances, which can often lead to cardiac arrest. In general, it is clear that the body needs it. It enters, in most cases (like other trace elements) with food.
Important! If necessary, clarify some data on certain trace elements / amino acids, etc., I use the database of the US Department of Agriculture (United States Department of Agriculture Agricultural Research Service, it is USDA) and I strongly advise you. Objective source, in my opinion, does not exist.
So, according to this database, in bananas about 358 mg of potassium per 100 g of product, only kiwi with its 522 mg of potassium has comparable "power" of the available tropical "guests". Everything else is quite rare (tamarind - 628 mg, avocado - 485 mg (not rare, it is often found in sushi), durian - 436 mg, guava - 417 mg, passion fruit - 348 mg). At the same time, compare with the relatives "at each exit from the metro" products: dill - 738 mg, spinach - 558 mg, parsley - 554 mg, cilantro - 521 mg, even forest sorrel and then 390 mg per 100 grams of the product. There are also vegetables in Coy: Brussels sprouts - 389 mg, pumpkin - 340 mg, black currants - 322 mg. So before the next "find% nutrient% in 60 seconds on a shelf with subtropical fruits," take a look at the USDA base, maybe everything is already in a carrot or zucchini ...
In any vegetable / fruit / herbs besides potassium, there are its isotopes . Stable are 39 K (93.08% of the total mass), 40 K (0.01% of the total mass, half-life 1.248 * 10 9 years), 41 K (6.91% of the total mass). All the rest live from hours to nanoseconds and break up:
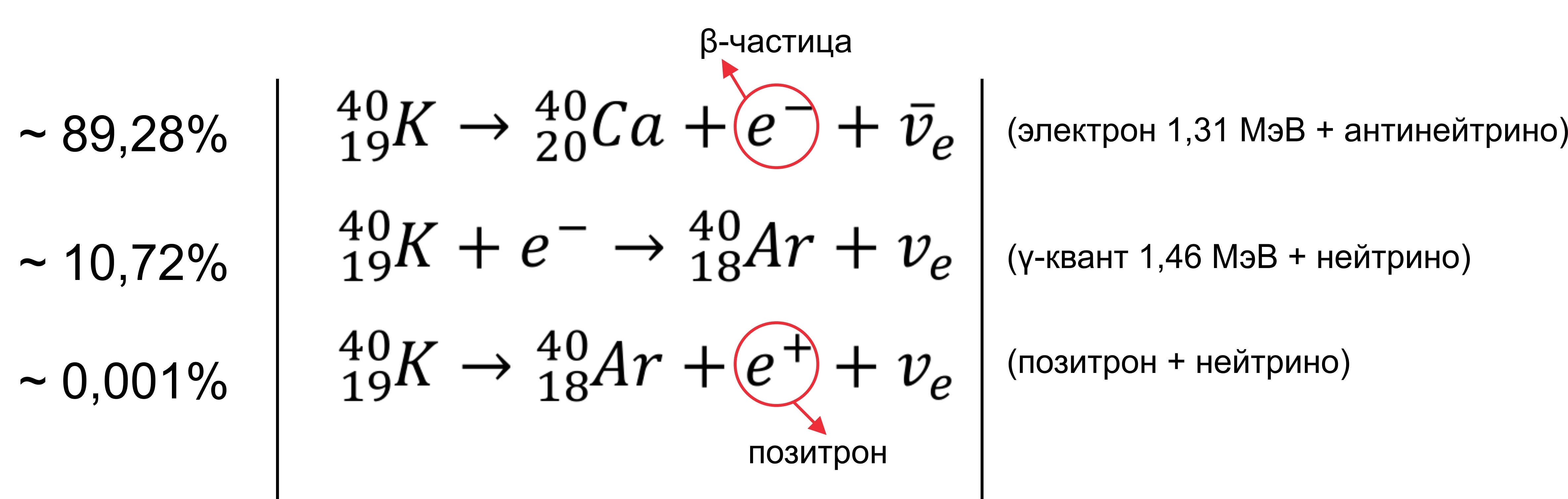
Our trace element (relative to the others) is unusual in that it has a 40 K isotope, which is a rare example of an isotope that undergoes both types of beta decay. In approximately 89.28% of cases, it disintegrates into calcium-40 ( 40 Ca) with the emission of beta particles (β - , electron) with a maximum energy of 1.31 MeV and antineutrino. About 10.72% of the time, it decays into argon-40 ( 40 Ar) by capturing electrons with the emission of gamma radiation with an energy of 1.460 MeV and neutrinos. The radioactive decay of this particular isotope explains the high argon content (almost 1%) in the earth’s atmosphere, as well as its high content compared to 36 Ar. Very rarely (in 0.001% of cases) it decays to 40 Ar, emitting a positron (β + ) and a neutrino. About the last reaction was written in the Habra article . Say banana source of antimatter.
Due to the facts, 40 K is the largest source of natural radioactivity of animals, including humans. In a gram of natural potassium, an average of 32 decays of potassium-40 occurs per second (32 becquerel, or 865 picocuries, or about one trillion parts of curie). The human body weighing 70 kg contains about 175 g of potassium, therefore, about 5,400 decays (≈ 5,400 Becquerel) occur every second, moreover, continuously throughout human life.
Becquerel (Russian designation: Bq; international: Bq) is a measure of the activity of a radioactive source in the International System of Units (SI). One becquerel is defined as the activity of a source in which, on average, 1 radioactive decay occurs in 1 second. The unit is named after the French scientist Antoine Henri Becquerel, one of the discoverers of radioactivity.
In principle, there is nothing surprising. In nature, there are more radioactive foods, moreover, radioactive, not only because of 40 K, but also, for example, radium (isotopes 226 Ra, 228 Ra). As an example, a Brazil nut is perfectly suited, the radioactivity of which can reach 12,000 picocuries per kilogram and above (450 Bq / kg and above).
To the note : smokers are the worst in this respect, since tobacco contains not only the already mentioned radium 226 Ra, but thorium 234 Th, polonium 210 Po and a whole lot more.
But for some reason, Comrade Gary Mansfield from the Livermore National Laboratory. Lawrence, doing the nuclear security newsletter of RadSafe in 1995, wrote about the "banana equivalent dose" and a new era began. The era of the radioactive banana (the banana equivalent is a much more vigorous thing than the banana argument described in the article ).
The equivalent dose of a banana (BED) is a completely unofficial unit that characterizes the effects of ionizing radiation. Its main purpose is to act as an even standard user available, with which doses of radioactivity can be easily compared. In fact, it is a tool for describing infinitely small doses of radiation (and infinitesimal risks for the population from them). Excerpt from Wikipedia (RU) :
... Since death or a serious illness caused by a low dose of radiation (below 0.5 Gy) is extremely rare, it turned out that it is impossible to link them with radiation exposure on the body with confidence - observations will be required for a long time (over 12 years) over a huge sample people exposed to such a dose. Moreover, the positive effects of low doses of radiation on living organisms, hormesis, were found. The phenomenon of mass consciousness is also associated with small doses of radiation, when uncertainty about the safety issue (or the certainty that the existing danger is insignificant) is interpreted as the deliberate presence of danger and a massive fear of small doses of radiation is formed.
A few words about radiation hormesis :
The term radiation hormesis was proposed in 1980 by T. D. Lucky and means the beneficial effects of low doses of radiation. The mechanism of radiation hormesis at the cell level of warm-blooded animals consists in initiating protein synthesis, gene activation, DNA repair in response to stress - the effect of a small dose of radiation (close to the value of the natural radioactive background of the Earth). This reaction ultimately causes activation of membrane receptors, proliferation of splenocytes and stimulation of the immune system. (1994 - Report of the UN International Committee on the Effects of Atomic Radiation).
Being a student of the Department of High Energy Chemistry, I respect the concept of hormesis (~ radiation hormesis) with respect (respect, in due time, was supported by experimental course work performed in one of the hospitals). IMHO small but constantly, more harmful than large, but once. A drop wears away a stone.
In order to better understand what a small dose and what is NOT a small one, you can use, in addition to the banana equivalent, a visual aid - a summary table of radiation doses ( increase ) created by the engineer and popularizer of science Randall Patrick Monroe (note my banana equivalent is outlined in red) .
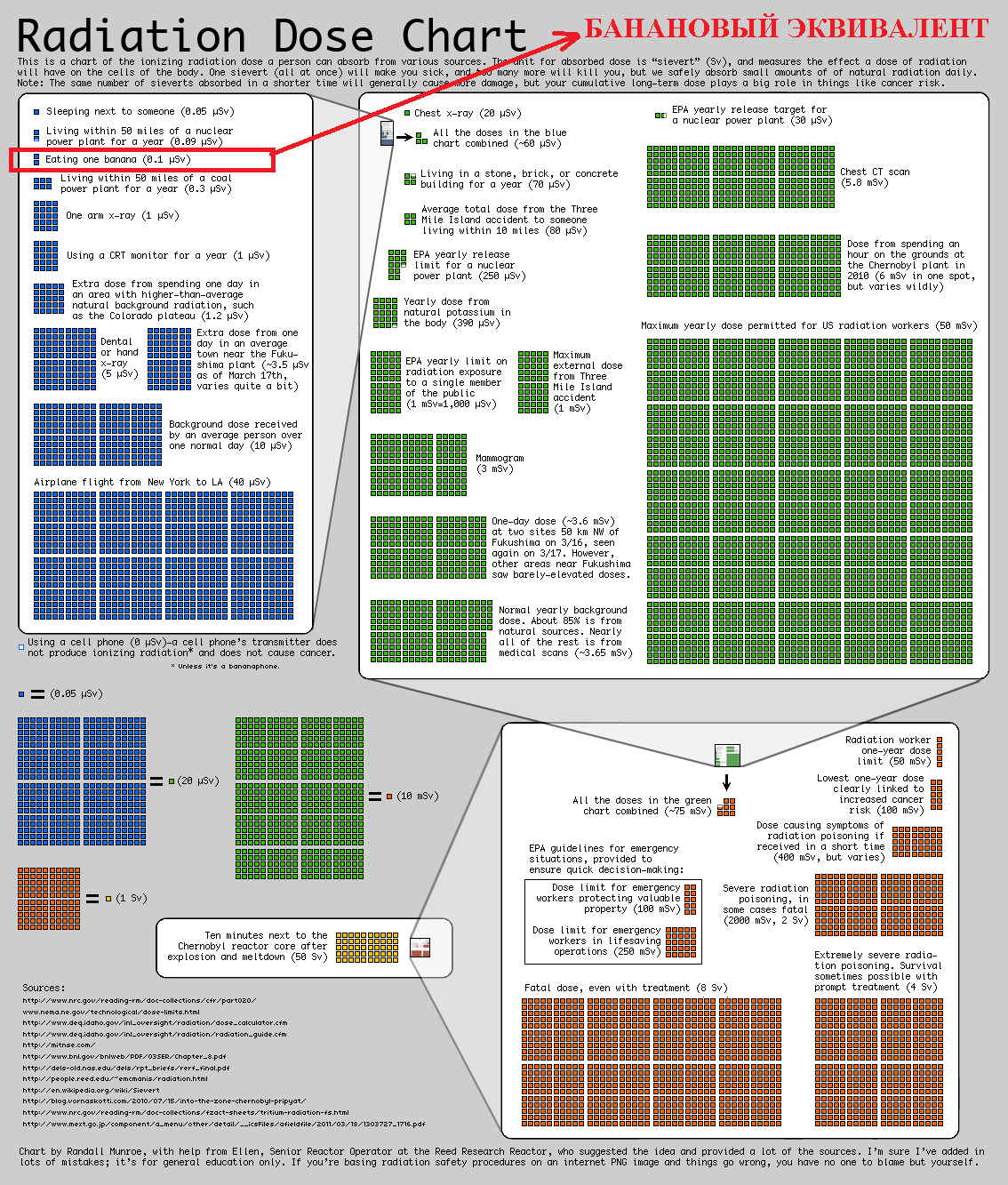
Well, if the table does not suit for some parameters, we return to our banana equivalent. 1 BED is approximately equal to the dose of radioactivity that a person receives by eating one medium-sized banana, weighing about 150 g (5.3 ounces) with an activity of isotopes of about 15 Bq . This is calculated all the way by multiplying the expected equivalent dose that an adult can grab for 50 years from a pure isotope 40 K by the isotope activity and by the mass of potassium in a banana. We get:
1 BED ≈ 5.02 nSv / Bq x 32 Bq / g x 0.537 g ≈ 86 nSv = 0.086 μSv (µSv) = 8.6 micro X-rays (μrem)
It is generally accepted to round this value to 0.1 µSv (10 micro-roentgens) to simplify calculations and ease of perception. In general, if you eat one average banana daily for a year, the total equivalent dose will be ≈ 37 μSv ≈ 3.7 mrem.
By the way, the expected equivalent dose (5.02 nSv / Bq) is taken from American sources ( EPA ). But the International Commission on Radiological Protection uses a different value for this coefficient = 6.2 nSv / Bq and then when recalculating the dial it will turn out not so beautiful. It will be more difficult to count, to represent scales, etc. Therefore, they use American data.
Note: i.e. theoretically, using the above formula can create your% VEGETABLE / FRUIT% equivalent relative to 40 K. For example, the average weight of a commodity tuber of a variety (a bag which Lukashenko gave Putin for the New Year) is 100 grams. We go to watch the base of the US Department of Agriculture on the fact of potassium content in potatoes. It is also important to choose the right option (with skin / without, etc.). Well, let it be on average 430 mg of potassium. We consider and get 6.9 microroentgen. Draw your own conclusions (or do not do it, but read on).
Why is the unit unofficial (and even comic)? And because the "external" potassium (and therefore its isotopes), which is ingested with food, does not accumulate in it (ie, the "banana dose" is not cumulative). This is due to the homeostasis of the human body.
Homeostasis (ancient Greek ὁμοιοστάσις from ὅμοιος “identical, similar” + στάσις “standing; stillness”) - self-regulation, the ability of an open system to maintain the constancy of its internal state through coordinated reactions aimed at maintaining dynamic equilibrium.
Those. any excess of the component, coming with food, is rather quickly compensated by the withdrawal of the same amount with secretions of the body. In fact, the additional radiation caused by consuming a banana lasts only a few hours after ingestion, that is, until the kidneys restore the normal amount of potassium in the body. Tells us about this and the document issued by the US Environmental Protection Agency. I will quote:
For radioisotopes of elements actively involved in the homeostasis of the human body, the correction factors for calculating the risk of inhalation or ingestion given in this document are not suitable for use in some cases. For example, the risk coefficient of ingestion for 40 K is not suitable for calculation when using natural products containing a high content of 40 K. This is due to the fact that the biokinetic model used in this document implies a relatively slow removal of this element (biological half-life of 30 days) that occurs when the average amount of potassium in the body. A sharp increase in the use of potassium with food leads to the elimination from the body of an equal mass of biogenic potassium (including the 40 K isotope) in a short period.
Plus, if the estimated time spent by a certain mass saturated with an isotope in the body decreases N times (due to the simultaneous intake of a laxative, for example), then the calculated equivalent absorbed dose decreases N times too.
So ... A much more harmful phenomenon, in my opinion, is the case when many small sources of radiation are combined (in storage or in warehouses). No wonder there are stories about the false positives of ionizing radiation sensors at US customs when cars loaded with bananas passed through a checkpoint.
I don’t know how many people know, but Canada, Belarus and Russia are the largest producers of potash fertilizers in the world (!). Most often, these fertilizers come in the form of KCl, K 2 SO 4 * MgSO 4 and rare KNO 3 potassium nitrate. And here the scale is far from banana. For example, in 1 kg of the most common potash fertilizer KCl (potassium chloride) ~ 524 grams of potassium, i.e. that's almost 1000 BED (a thousand bananas). Naturally, no one in their right mind will eat this fertilizer, nor will it, because about 15 grams inside can easily lead to cessation of heart contractions. But on the other hand, he often saw, especially during the spring sowing campaign in Belarus, the men who adjoined to rest on bags of fertilizers.

Roughly speaking, he stuffs electrons (we do not take into account the decay with the release of a gamma quantum) back rather quickly (about the effects on the body, thanks to the Javian specification, can be read here ). Polyethylene bag will not save . Below is a picture for those who forgot the lessons of GO (or who simply did not have them :()
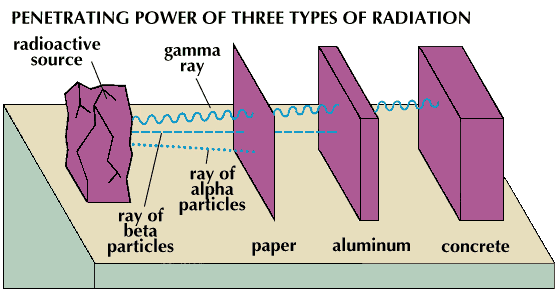
Beta particles (electrons) can more or less be absorbed by only a few millimeters of aluminum. Wrap yourself with foil before you lie down or something ...
In conclusion, as always, a small laboratory work on the subject “we study the dosimeter”. The pictures are a comparison of bananas and some salts containing potassium.

This is a pot with Chinese KOH (potassium hydroxide). I think the pipe cleaning agent "Mole" is spinning electrons like (if KOH is used there, and not cheaper NaOH)

It gives such a background

But such values in Chinese KCl (potassium chloride)

Well, talking about salt would be incomplete, if you do not mention KBr (the same sedative who is allegedly fed to soldiers in the barracks to reduce libido), the Soviet production is still

The difference, as they say, is visible to the naked eye. So that...
The moral of the note is that the banana radioactivity = the 40 K radioactive isotope that exists for millennia. If you come from the constellation of Sirius (and all the dogons can confirm this) with a different level of background radiation, you will have to give up bananas (and Belarusian potatoes, by the way). the rest - "do not think about it." Smoking, by the way, harms much more (for objective reasons, such as the fact that the gamma radiation that occurs during the decay of the isotopes present in tobacco penetrates more than any kind of electron from a banana). Well ... beware of a long stay near large clusters of bananas / potassium salts, etc. sources 40 K.
Sergey Besarab ( Siarhei V. Besarab )
Under the spoiler comments from Sumah about the banana equivalent
Banana equivalent slyness
- Potassium-40 is high energy with all that it implies. radiation energy can be different for different isotopes. if instead of potassium there is an isotope with low / medium energies, the harm will not necessarily be the same or less. At short distances inside the body, the question is whether the high or low energies are worse / better.
- Banana equivalent considerations relate mainly to external exposure. The Taiwanese example is external exposure. Residents of the building did not inhale cobalt, did not eat cobalt. But even with external irradiation, it is necessary to take into account that the energies are different.
- The argument about the banana equivalent as the equivalent of internal exposure is also very strange. Different isotopes have different half-life from the body. In our environment there are isotopes, the excretion of which is not proven. So, at any concentration of these isotopes in the medium, they accumulate. Those. threshold for does not exist. How much the organism pulls from the environment of this% @ # ma will remain so much in the grave and / or partially fly into the crematorium pipe.
- No one has canceled the different radiotoxicity of isotopes. SanPins nobody canceled.
- No one has canceled the local concentration of radioactive isotopes in the body compared to the habitat, which can differ by 10 or 100 times. No one has canceled the local concentration in the body, which may also differ by orders of magnitude.
- The energy of potassium-40 and the energy of cosmic radiation (in an airplane) are also, as it were, different energies.
- About examples with a dosimeter. Gas discharge dosimeters overestimate the dose rate while in the high energy field. Dose rate cannot be a measure of safety.
About the visibility of potassium dosimeter in food and other "miracles" associated with the detection of radioactive isotopes in food and in the environment.
If you use a cesium iodine scintillation detector with a size of 5 5 30 and a lead chamber with a wall thickness of 6 mm (chamber bottom 12 mm), then the results will be as follows with a statistical error of 2% and one sigma (68% confidence interval):
- with a natural background of about 10 μR / h inside the empty lead chamber, the scintillator counts 0.070 μR / h.
- if you put a small sample of the product in the chamber, for example, pour the zip-bag not the fattest drinking yoghurt, then the dosimeter counts about 0.080 μR / h or more on the bag's surface.
The difference in readings will be due to the influence of potassium.
The irony is that in yogurt at this moment there can be cesium-137 at the level of Becquerels per liter, but it will not appear at all. Those. radiation from radioactive potassium will completely mask radiation from radioactive cesium-137.
Dixy stores sell strawberry yogurt in a 0.5 liter tetrapack with cesium-137 content of about 4Bq / liter. Yoghurt is produced in the settlement Starodub of the Bryansk region. Starodub settlement is located on the border of a fairly significant Chernobyl spot in this region. Well, the Bryansk exclusion zone is not very far from Starodub.
4Bq / l is, of course, lower than the cesium-137 rationing, but if you sit on the cesium-137 substrate continuously, then arguments about the half-life of cesium-137 from the body become inappropriate to explain the safety of such consumption.
In addition, we can assume that cesium-137 is a marker of radioactive contamination, about which we know little.
There are many examples when the gamma spectrometer does not detect the peak of cesium-137 in the air, but detects a cacophony in the low-energy region. At the same time, in the sample of surface soil or fungus / berry, the gamma spectrometer from man-made isotopes detects only traces of cesium-137. Moreover, this cesium can be 50 Bq / kg or more.
How to increase the level of detection "in everyday life", if this is even possible?
- You can somehow increase the proportion of radioactive substances: sublimate a sample of the product, for example.
- if there is a scintillator, you can use a lead chamber or screen in the form of a thick layer of water or snow, shielding radiation from the ground.
- if a face dosimeter, then a lead chamber.
- if the gas-discharge dosimeter, the sample sublimation. Screens can also be used, but the gas discharger is very sensitive to cosmic radiation. it will periodically spoil the picture more often than the required exposure time. in principle, if you record a dose rate graph, you can simply try to cut out points that are similar to the reaction to cosmic radiation, but these are all dances with tambourines. but the screen allows the gas discharger to assess the situation quickly. the drop in dose rate over the screen will still be. Concrete, by the way, shields well, if the gravel inside the concrete does not glow strongly.
But of all of the above, the scintillator and swine chamber are the most efficient and fast. To get to the results of 0.070 μR / h with an accuracy of 2% you need two hours of exposure
')
Source: https://habr.com/ru/post/435854/
All Articles
