May the force be with us: our own immunity against cancer
Over the past decades, science has noticeably advanced in the treatment of cancer, and although we are still quite far from complete victory over this terrible disease, doctors have more and more tools to destroy tumors or limit their growth. The main thing - they give cancer patients the opportunity to live longer.
One of these tools is the activation of a person’s own immunity against cancer cells. There is a whole direction dedicated to this - immuno-oncology. A lot of attention is focused on it, it is in this area that the most research is being conducted today and the most promising medicines are being developed.
We in “Medicine 24/7” actively use immunotherapy - and we see that it gives good results. True, we are confronted with the fact that many patients are completely unaware of this method of treatment or consider it to be insufficiently studied and not trustworthy.
In this publication we will try to clarify the questions: what is immunotherapy, how it works and who can help.
')

Judy Perkins. She had end-stage breast cancer, which was completely cured with the newest method of immunotherapy.
Hidden threat. How does cancer arise
Cancer cells are mutant rebels who managed to outwit the system.
In the process of life, all the cells of the body undergo strictly defined stages of development, perform specified functions, multiply according to strict rules, and eventually grow old and die. This is a natural process. The programmed death of old cells that have accumulated a lot of damage is called apoptosis.
However, under the influence of heredity or adverse external factors, some cells accumulate genetic errors and "rebel": they refuse to live according to the nature of the algorithm, start to multiply uncontrollably, or do not die in time. This is not uncommon. Potentially cancer cells can periodically appear in each - this is normal. Almost always such "upstarts" are killed by the internal security service of the body - immunity .
One of the main roles in this process is played by T-lymphocytes, or, more simply, T-cells . They react to an antigen (a substance foreign to the body), recognize and destroy potential enemies: for example, microbes or inappropriate donor material. Normally, T-lymphocytes kill and body cells that have begun to mutate and behave not according to the rules. Therefore, cancer does not occur in all - for most immunity copes with the disorders before they spread.
But cancer tends to survive and the tumor cells try to capture as many resources as possible, to become "more successful." They multiply faster, secrete vascular growth factor (to attract more blood and nutrients to the tumor), develop resistance to drugs, force stem cells to increase the growth of tumor tissues (sending fraud signals with a request for regeneration).
Cancer cells achieve special success in masking: some of them remove special antigen-proteins from their surface, by which T-cells can recognize them. Others secrete special molecules that suppress immunity, and some even form hybrids with macrophages (one of the types of immune cells) - and literally acquire superpowers!
In this, they are helped, on the one hand, by their relationship with the normal cells of the body — a kind of innate disguise. On the other hand, the genetic variability of cancer cells gives them increased adaptability. The more mutations accumulated in the cell's DNA at the time of its malignancy (transformation into a malignant one), the more chances it has to go through the immune response and develop a successful capture plan.
Awakening of power. The history of the Nobel discoveries
Human immunity is actually a real army of ruthless murderers, and after each “military operation” to neutralize the next enemy, they must be calmed down and transferred from military to peace. This mechanism reduces the temperature to normal values and stops inflammation when the danger has passed and the infection is defeated.
The Nobel Prize in Physiology and Medicine in 2018 was awarded to the American James Ellison and the Japanese Tasuku Hongjo for their independent discoveries in the same field: exactly how does this switch from aggressive to calm mode.
None of the scientists at first thought about cancer treatment. Both of them wanted to understand the work of the immune response more clearly. By that time, it was clear that both on the surface of T-cells and on the surface of antigen-presenting cells ( APC ) there are receptor molecules that act on each other, provoking or slowing down the work of the immune system. The TCR, a T-cell receptor by which T-cells recognize "enemy" proteins exposed to APC, was discovered. We found the main histocompatibility complex MHC (major histocompatibility complex), with the help of which APC is delivered to the identification of T-cells pieces of foreign proteins. Peter Doherty and Rolf Zinkernagel won their Nobel Prize for discovering this scenario in 1996.
Scientists understood that receptors on the surface of T cells work in conjunction with co-stimulants on the surface of APC. The CD28 protein from the surface of T cells was isolated in 1980 , and soon the B7 molecule was found on the surface of APC. In the course of the experiments, the researchers of the Ellison group transferred the B7 gene to cancer cells, and they began to be rejected by healthy tissue. It turned out that B7 binds to CD28 on the T-cell, and thus starts its work: the T-cell destroys the tumor cell, on the surface of which the B7 protein “sticks out”.
In 1987 , Allison discovered cytotoxic T-lymphocytic antigen-4 CTLA-4 (cytotoxic T-lymphocyte-associated antigen-4) - and found that this protein is similar in structure to the long-known CD28, and is also able to bind to B7 - however, when This works the opposite way: it stops the immune response.
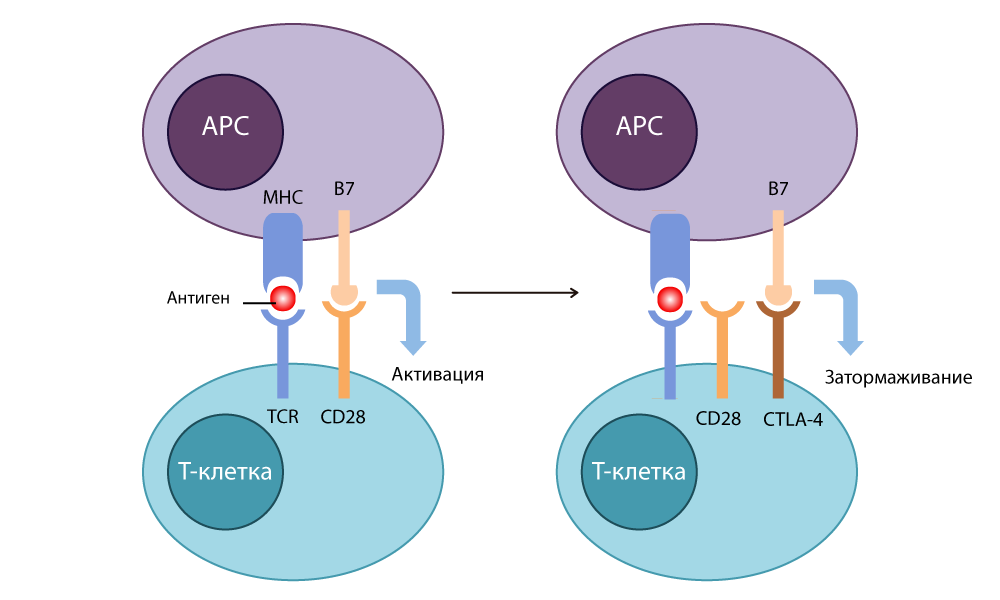
CTLA-4 action
At first, doctors were going to use this “brake” to fight autoimmune diseases (when the immune system starts attacking healthy cells of the body). But Allison came up with a brilliant thing: do not put pressure on the brake, but turn it off.
He developed an antibody-inhibitor (switch) that bound to CTLA-4 and prevented him from linking with B7 to disable immune responses. The free B7 molecules contacted CD28, the T-cell was activated and was again ready to kill. When in 1995 he conducted experiments on cancer patients in mice, it became clear that even cunning cancer cells could not hide from such T-lymphocytes with disabled brakes. In 2010, successful studies have been conducted on hopeless patients. In some patients, melanoma disappeared along with metastases - an incredible result!
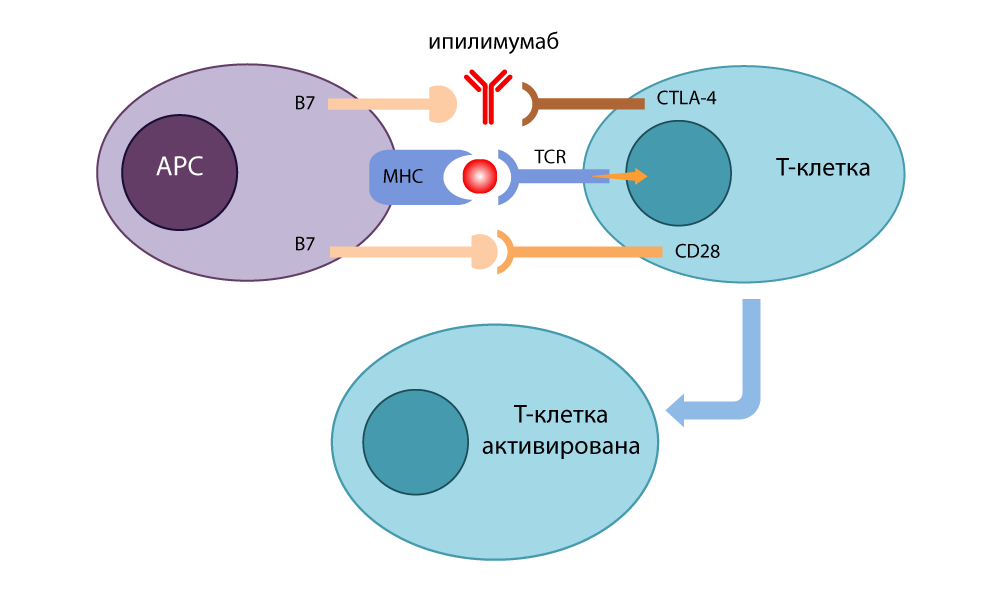
Action of the CTLA-4 inhibitor - ipilimumab
At the same time, in Kyoto, Tasuku Honjo found another receptor molecule on the surface of T-cells: PD-1 (Programmed cell Death protein-1 , Programmable Cell Death Protein-1). During the experiments (again on long-suffering mice), the Japanese found out that turning off the gene encoding this protein provokes symptoms of autoimmune disease in mice - that is, inhibition of PD-1 also turned off the “brakes” in T-lymphocytes and made them aggressive and active.
Honjo found out that PD-1 puts the T-cell into “sleep mode” when it binds to PD-L1 / PD-L2 protein on the surface of the antigen-presenting cell (APC). The PD-1 inhibitor opened this link and re-activated T cells. The effect of this “brake” was similar to the action of CTLA-4, but it took a different route.
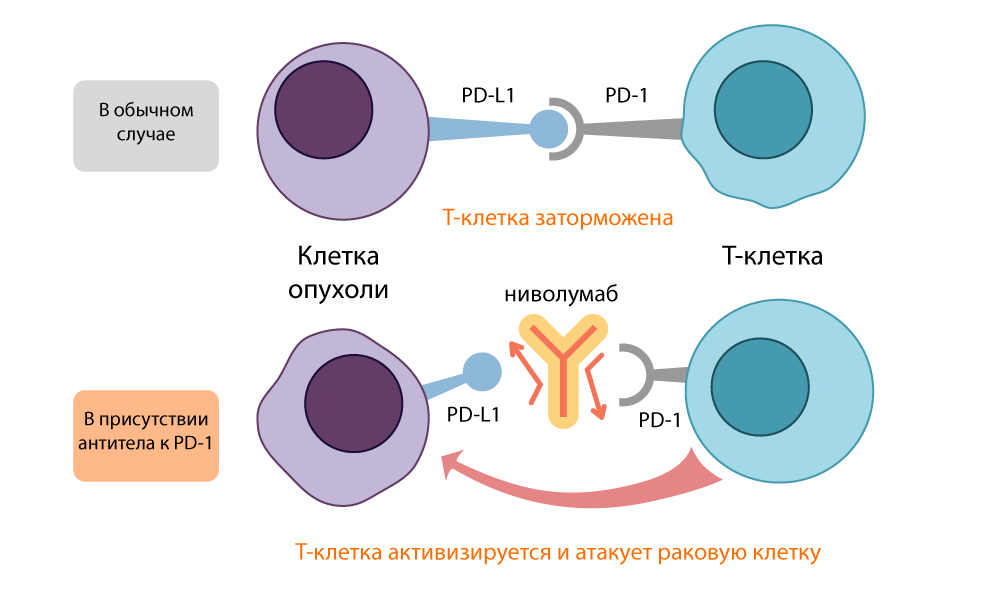
Action of PD-L1 inhibitor - nivoluumab
Both open inhibitory molecules, CTLA-4 and PD-1, were called immune control points (checkpoints) - it was their number and activity that made T-cells make a decision: calm down or start to fight.
It turned out that CTLA-4 blockers activate immunity in general, all T-cells, and the PD-1 inhibitor acts more specifically on tumors, since many cancer cells carry the “second piece of the puzzle,” the PD-L1 / PD-L2 molecules. Because of this, treatment with PD-1 inhibitors results in less risk of complications.
Immunity strikes back. What do control point inhibitors help
Allison and Honjo made not only a serious contribution to the understanding of physiological processes, but also launched a wave of fundamentally new practical research in applied medicine.
The discovery of inhibition of immune checkpoints (ICT) opens up a fundamentally new area of search for solutions. Previously existing methods of fighting cancer: surgery, radiation and chemotherapy - were directed directly to the tumor itself, to the destruction of cancer cells. Now physicians have a huge field for research in a completely different direction: changing the interaction of cancer cells with their environment.
By the way, it was this fundamental difference that gave physicians a real breakthrough. Until now, the tumor acted depending on its location. For breast cancer, one drug, for stomach cancer, a very different one. In 2017, the ICT inhibitor pembrolizumab was registered for the first time in the history of oncology as a drug for treating any cancer in any organ - if only tests confirm that the tumor has a special property: microsatellite instability. That is, its DNA is particularly prone to mutations. Previously, it has never been possible to make a cure for cancer on some common basis. This is a great achievement.
The revolution was the results of the use of new drugs against the most aggressive types of cancer: metastatic melanoma at the IV stage was considered incurable. And patients with such a diagnosis who underwent a course of the drug ipilimumab (CTLA-4 blocker) in 2010 received an additional year of life, so the development of the tumor stopped. In 58% of them, the tumor has decreased by a third.
In the treatment of non-small cell lung cancer nivolumab (PD-1 inhibitor), the risk of death of patients decreased by 40%.
The drug pembrolizumab (also an inhibitor of PD-1) showed a decrease in tumor growth by 43% in the group treated with melanoma. 74% of patients lived without deterioration during the year, for 18 months there were 71%. It is important that the effect of prescribing the drug outweighed the side effects at all stages of the disease.
Today, CTLA-4 and PD-1 inhibitors are used to treat melanoma (including inoperable), non-small cell lung cancer, squamous cell head and neck cancer, renal cell carcinoma, certain types of lymphomas, rectal cancer, bladder cancer, and tumors with microsatellite instability.
Special attention is attracted to studies that show the effectiveness of combination therapy with both anti-PD-1 and anti-CTLA-4 drugs.
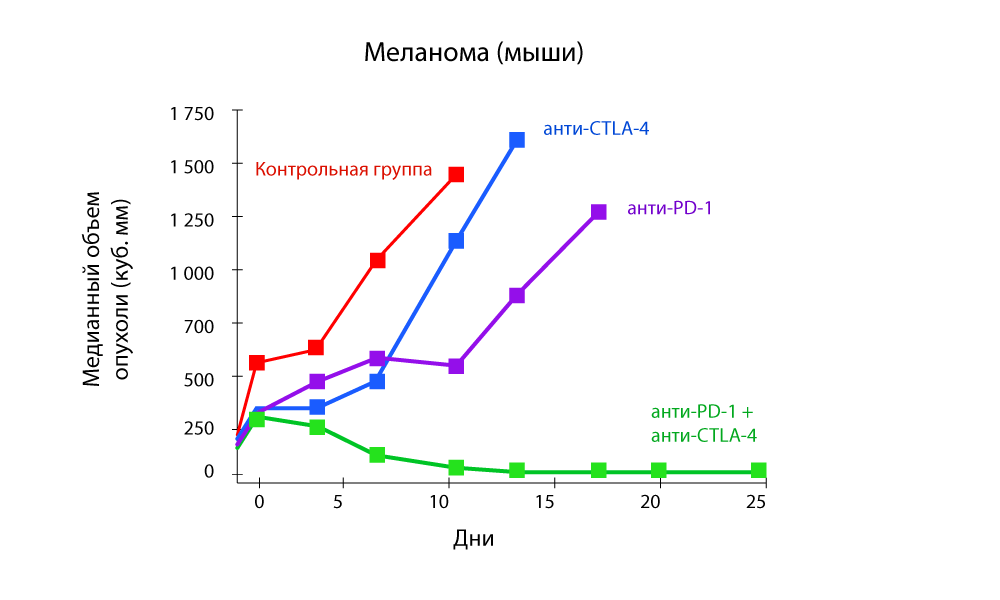
The change in tumor volume - a sharp decrease in the combination of anti-PD-1 and anti-CTLA-4 drugs
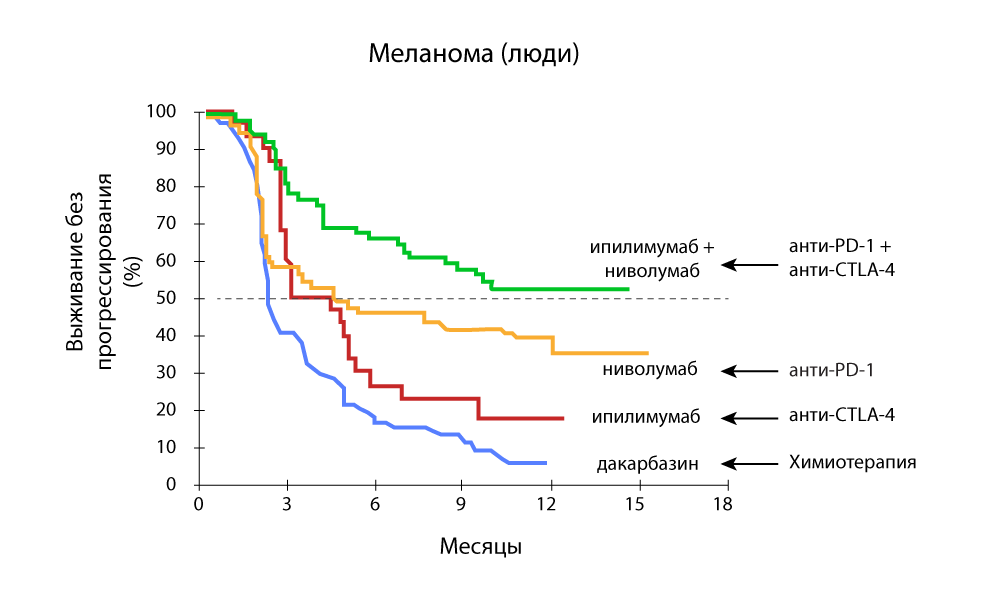
Progression-free survival - a combination of anti-PD-1 and anti-CTLA-4 drugs is more effective.
In Medicine 24/7, we successfully use pembrolizumab and nivoluumab from the moment they are registered in the Russian Federation. We followed all foreign studies and were very much looking forward to replenishing the arsenal.
Attack of the clones. Genetically modified immunity
Immune control points inhibitors are deservedly in the center of attention, but this mechanism is still not perfect and cannot cure any cancer. It is good that related areas of research are actively developing in immunotherapy. One of the most promising is CAR-T therapy .
The letter T in the name of the method is the same invariable T-cells of our immunity. The CAR (Chimeric antigen receptor) is a chimeric antigen receptor. Why is the receptor called chimeric? Because it is assembled from several parts taken from different cells - using the skills of genetic engineers.
A normal T cell has a special TCR receptor (T-cell receptor) . He "feels" all the cells of the body in his path and, if he senses some foreign molecule on the cell surface, sends an activating signal to the T-cell. She, in turn, either cracks down on an unwanted newcomer herself, or secretes special active substances (cytokines) and calls on other cells of the immune system to "sort things out". They kill T cells very effectively.
True, not very accurate. There are far fewer TCR species than antigens exist. Therefore, T cells are able to recognize many antigens by their TCR, but only approximately. Cancer cells often take advantage of this weakness in our security system and pretend to be "their own."
Evolution solved the problem as best it could: in the human body there is another mechanism for identifying outsiders: antibodies . These are special proteins that are secreted by another class of immune cells: B-lymphocytes. In B-cells, in contrast to T-cells, each “client” has an individual approach.
The antibody is a protein structure in the form of a letter Y. At both ends of this "fork" there are sites that bind to the antigen. These sites may change with each next generation of antibodies to fit snugly to the antigen — like picking puzzle pieces. When a foreign antigen is detected, B cells secrete billions of antibodies, among which there is a selection for the most accurate match for the antigen. As a result, reference antibodies are obtained, “trained” especially for very precise recognition of a specific “alien” antigen.
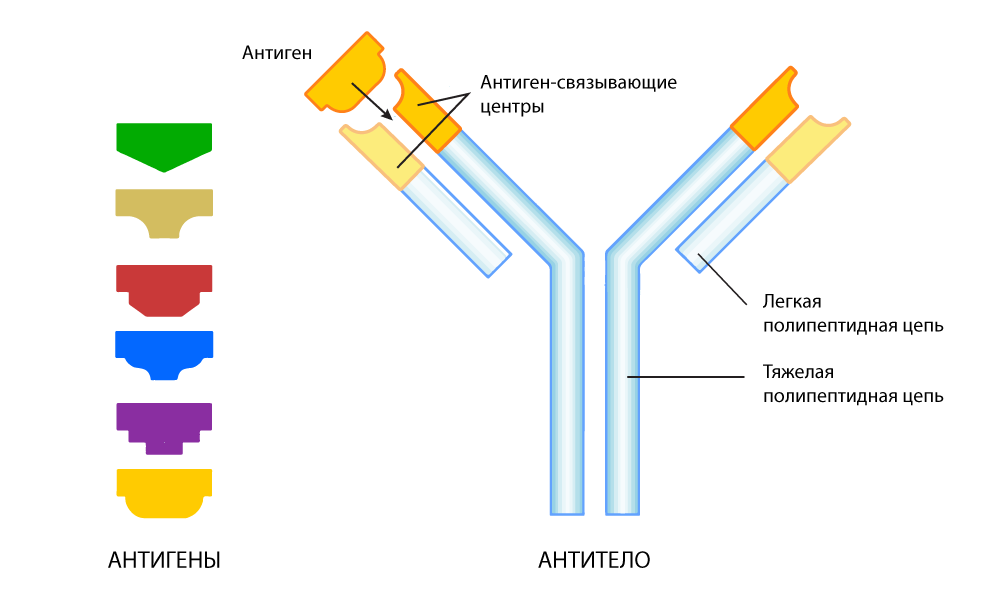
Antibody adapted to find a specific antigen
However, recognizing does not always mean disabling. With this, the complexity of antibodies - independently destroy the "enemy", they can not in all cases.
So, in 1989, an Israeli chemist and immunologist Zelig Ashhar came up with the idea of combining the deadly power of weak-sighted T-cells and the sniper aiming of antibodies. He singled out the end sections of protein-antibodies that are able to tightly bind to the antigen of certain cancer cells, and “transplanted” them into a T-cell — they replaced part of the TCR responsible for the recognition of antigens.
Subsequently, he began working together with his American colleague, Stephen Rosenberg, they managed to make the chimeric receptor more efficient design, at the same time sensitive and selective.
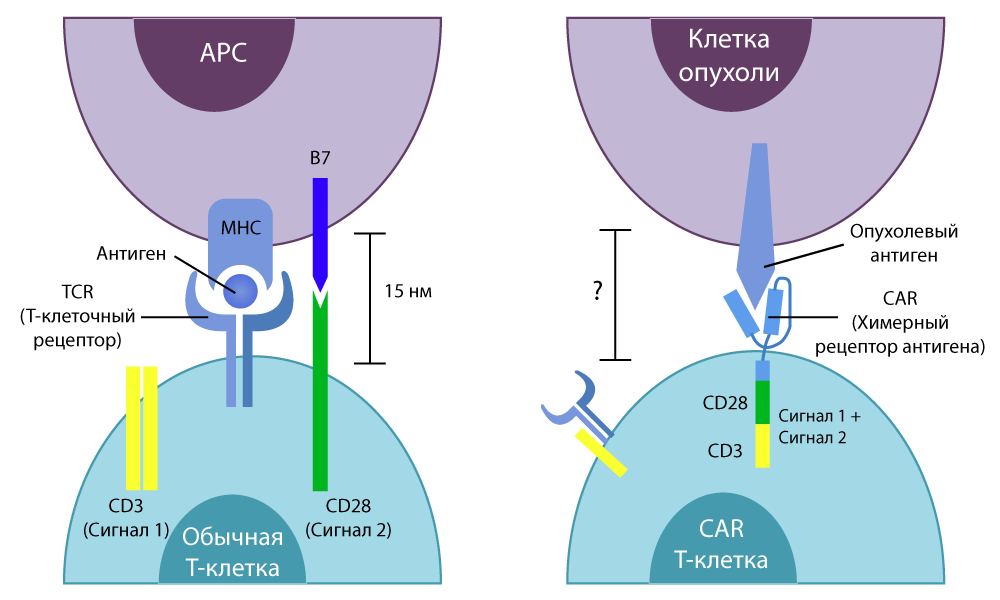
The difference between normal T-cells and CAR-T-cells
In vitro studies have shown good results. Then the scientists again treated the mice, then painstakingly transferred the technique to humans.
Over time, CAR-T therapy has led to a modern look.
In the clinical trials started in 2010, encouraging results were obtained immediately: in the treatment of lymphoma, 12 out of 13 patients showed improvement, and 4 had remission. In the treatment of leukemia, remission occurred in 17 out of 33 people.
In 2018, Nature Medicine published an article by American oncologists, where it was reported that they had been observing a patient completely healthy for two years after CAR-T therapy. She was cured of metastatic breast cancer with metastases. This picture of her in a kayak is given at the beginning of the article: after treatment she returned to work and goes on hikes.
New Hope. Will immunotherapy be a panacea?
Like other cancer treatments, immunotherapy has its limitations. Despite the fact that in some cases patients give a very good response to therapy with inhibitors of immune control points, in 60% of cases either the acquired resistance develops or primary resistance to anti-PD-1 or anti-CTLA-4 drugs is observed: the tumor simply does not respond for treatment or quickly adapts and learns to "bypass" it.
In addition to PD-1, PD-L1 / 2, CTLA-4, CD28 and B7, there are a lot of other co-receptors on the surfaces of T-cells and tumor cells, the effect of which has not yet been studied as well as the work of control points, but they also affect on the immune response. One of the areas of work is the effect on these co-receptors.
In addition, ICT therapy is complemented by the introduction of vaccines, cytokines, beta-blockers - and this approach also works well in some cases .
CAR-T therapy is still extremely expensive and is still only entering the stage of commercial use: research is being carried out in scientific groups of Ashhar and Rosenberg, other researchers - each of the groups creates special types of CAR-T with directed action against a certain type of cancer. But for now this is only research, testing and testing. It will take several years before it turns into a spent mass treatment method - but even then it will not be possible to give 100% guarantees.
But while scientists are conducting research, doctors are introducing experimental treatment regimens using those achievements that already exist. And the most noticeable effect is given by the combination of immunotherapy with the classic "three pillars" of oncology: radiation and chemotherapy, surgery. When combining these methods, synergy is always obtained: together they work more efficiently than in turn.
, ( ) , , , : .
. , , . , , , , . . , 50% . , .
. 3 , 3 «», , , .
– . , , . , 2-3 . , , , . , .
. « 24/7» , , - . , . – . – . III-IV . , , .
, – . , , , . , , . , . .
One of these tools is the activation of a person’s own immunity against cancer cells. There is a whole direction dedicated to this - immuno-oncology. A lot of attention is focused on it, it is in this area that the most research is being conducted today and the most promising medicines are being developed.
We in “Medicine 24/7” actively use immunotherapy - and we see that it gives good results. True, we are confronted with the fact that many patients are completely unaware of this method of treatment or consider it to be insufficiently studied and not trustworthy.
In this publication we will try to clarify the questions: what is immunotherapy, how it works and who can help.
')

Judy Perkins. She had end-stage breast cancer, which was completely cured with the newest method of immunotherapy.
Hidden threat. How does cancer arise
Cancer cells are mutant rebels who managed to outwit the system.
In the process of life, all the cells of the body undergo strictly defined stages of development, perform specified functions, multiply according to strict rules, and eventually grow old and die. This is a natural process. The programmed death of old cells that have accumulated a lot of damage is called apoptosis.
However, under the influence of heredity or adverse external factors, some cells accumulate genetic errors and "rebel": they refuse to live according to the nature of the algorithm, start to multiply uncontrollably, or do not die in time. This is not uncommon. Potentially cancer cells can periodically appear in each - this is normal. Almost always such "upstarts" are killed by the internal security service of the body - immunity .
One of the main roles in this process is played by T-lymphocytes, or, more simply, T-cells . They react to an antigen (a substance foreign to the body), recognize and destroy potential enemies: for example, microbes or inappropriate donor material. Normally, T-lymphocytes kill and body cells that have begun to mutate and behave not according to the rules. Therefore, cancer does not occur in all - for most immunity copes with the disorders before they spread.
But cancer tends to survive and the tumor cells try to capture as many resources as possible, to become "more successful." They multiply faster, secrete vascular growth factor (to attract more blood and nutrients to the tumor), develop resistance to drugs, force stem cells to increase the growth of tumor tissues (sending fraud signals with a request for regeneration).
Cancer cells achieve special success in masking: some of them remove special antigen-proteins from their surface, by which T-cells can recognize them. Others secrete special molecules that suppress immunity, and some even form hybrids with macrophages (one of the types of immune cells) - and literally acquire superpowers!
In this, they are helped, on the one hand, by their relationship with the normal cells of the body — a kind of innate disguise. On the other hand, the genetic variability of cancer cells gives them increased adaptability. The more mutations accumulated in the cell's DNA at the time of its malignancy (transformation into a malignant one), the more chances it has to go through the immune response and develop a successful capture plan.
Awakening of power. The history of the Nobel discoveries
Human immunity is actually a real army of ruthless murderers, and after each “military operation” to neutralize the next enemy, they must be calmed down and transferred from military to peace. This mechanism reduces the temperature to normal values and stops inflammation when the danger has passed and the infection is defeated.
The Nobel Prize in Physiology and Medicine in 2018 was awarded to the American James Ellison and the Japanese Tasuku Hongjo for their independent discoveries in the same field: exactly how does this switch from aggressive to calm mode.
None of the scientists at first thought about cancer treatment. Both of them wanted to understand the work of the immune response more clearly. By that time, it was clear that both on the surface of T-cells and on the surface of antigen-presenting cells ( APC ) there are receptor molecules that act on each other, provoking or slowing down the work of the immune system. The TCR, a T-cell receptor by which T-cells recognize "enemy" proteins exposed to APC, was discovered. We found the main histocompatibility complex MHC (major histocompatibility complex), with the help of which APC is delivered to the identification of T-cells pieces of foreign proteins. Peter Doherty and Rolf Zinkernagel won their Nobel Prize for discovering this scenario in 1996.
Scientists understood that receptors on the surface of T cells work in conjunction with co-stimulants on the surface of APC. The CD28 protein from the surface of T cells was isolated in 1980 , and soon the B7 molecule was found on the surface of APC. In the course of the experiments, the researchers of the Ellison group transferred the B7 gene to cancer cells, and they began to be rejected by healthy tissue. It turned out that B7 binds to CD28 on the T-cell, and thus starts its work: the T-cell destroys the tumor cell, on the surface of which the B7 protein “sticks out”.
In 1987 , Allison discovered cytotoxic T-lymphocytic antigen-4 CTLA-4 (cytotoxic T-lymphocyte-associated antigen-4) - and found that this protein is similar in structure to the long-known CD28, and is also able to bind to B7 - however, when This works the opposite way: it stops the immune response.

CTLA-4 action
At first, doctors were going to use this “brake” to fight autoimmune diseases (when the immune system starts attacking healthy cells of the body). But Allison came up with a brilliant thing: do not put pressure on the brake, but turn it off.
He developed an antibody-inhibitor (switch) that bound to CTLA-4 and prevented him from linking with B7 to disable immune responses. The free B7 molecules contacted CD28, the T-cell was activated and was again ready to kill. When in 1995 he conducted experiments on cancer patients in mice, it became clear that even cunning cancer cells could not hide from such T-lymphocytes with disabled brakes. In 2010, successful studies have been conducted on hopeless patients. In some patients, melanoma disappeared along with metastases - an incredible result!

Action of the CTLA-4 inhibitor - ipilimumab
At the same time, in Kyoto, Tasuku Honjo found another receptor molecule on the surface of T-cells: PD-1 (Programmed cell Death protein-1 , Programmable Cell Death Protein-1). During the experiments (again on long-suffering mice), the Japanese found out that turning off the gene encoding this protein provokes symptoms of autoimmune disease in mice - that is, inhibition of PD-1 also turned off the “brakes” in T-lymphocytes and made them aggressive and active.
Honjo found out that PD-1 puts the T-cell into “sleep mode” when it binds to PD-L1 / PD-L2 protein on the surface of the antigen-presenting cell (APC). The PD-1 inhibitor opened this link and re-activated T cells. The effect of this “brake” was similar to the action of CTLA-4, but it took a different route.

Action of PD-L1 inhibitor - nivoluumab
Both open inhibitory molecules, CTLA-4 and PD-1, were called immune control points (checkpoints) - it was their number and activity that made T-cells make a decision: calm down or start to fight.
It turned out that CTLA-4 blockers activate immunity in general, all T-cells, and the PD-1 inhibitor acts more specifically on tumors, since many cancer cells carry the “second piece of the puzzle,” the PD-L1 / PD-L2 molecules. Because of this, treatment with PD-1 inhibitors results in less risk of complications.
Immunity strikes back. What do control point inhibitors help
Allison and Honjo made not only a serious contribution to the understanding of physiological processes, but also launched a wave of fundamentally new practical research in applied medicine.
The discovery of inhibition of immune checkpoints (ICT) opens up a fundamentally new area of search for solutions. Previously existing methods of fighting cancer: surgery, radiation and chemotherapy - were directed directly to the tumor itself, to the destruction of cancer cells. Now physicians have a huge field for research in a completely different direction: changing the interaction of cancer cells with their environment.
By the way, it was this fundamental difference that gave physicians a real breakthrough. Until now, the tumor acted depending on its location. For breast cancer, one drug, for stomach cancer, a very different one. In 2017, the ICT inhibitor pembrolizumab was registered for the first time in the history of oncology as a drug for treating any cancer in any organ - if only tests confirm that the tumor has a special property: microsatellite instability. That is, its DNA is particularly prone to mutations. Previously, it has never been possible to make a cure for cancer on some common basis. This is a great achievement.
The revolution was the results of the use of new drugs against the most aggressive types of cancer: metastatic melanoma at the IV stage was considered incurable. And patients with such a diagnosis who underwent a course of the drug ipilimumab (CTLA-4 blocker) in 2010 received an additional year of life, so the development of the tumor stopped. In 58% of them, the tumor has decreased by a third.
In the treatment of non-small cell lung cancer nivolumab (PD-1 inhibitor), the risk of death of patients decreased by 40%.
The drug pembrolizumab (also an inhibitor of PD-1) showed a decrease in tumor growth by 43% in the group treated with melanoma. 74% of patients lived without deterioration during the year, for 18 months there were 71%. It is important that the effect of prescribing the drug outweighed the side effects at all stages of the disease.
Today, CTLA-4 and PD-1 inhibitors are used to treat melanoma (including inoperable), non-small cell lung cancer, squamous cell head and neck cancer, renal cell carcinoma, certain types of lymphomas, rectal cancer, bladder cancer, and tumors with microsatellite instability.
Special attention is attracted to studies that show the effectiveness of combination therapy with both anti-PD-1 and anti-CTLA-4 drugs.

The change in tumor volume - a sharp decrease in the combination of anti-PD-1 and anti-CTLA-4 drugs

Progression-free survival - a combination of anti-PD-1 and anti-CTLA-4 drugs is more effective.
In Medicine 24/7, we successfully use pembrolizumab and nivoluumab from the moment they are registered in the Russian Federation. We followed all foreign studies and were very much looking forward to replenishing the arsenal.
Attack of the clones. Genetically modified immunity
Immune control points inhibitors are deservedly in the center of attention, but this mechanism is still not perfect and cannot cure any cancer. It is good that related areas of research are actively developing in immunotherapy. One of the most promising is CAR-T therapy .
The letter T in the name of the method is the same invariable T-cells of our immunity. The CAR (Chimeric antigen receptor) is a chimeric antigen receptor. Why is the receptor called chimeric? Because it is assembled from several parts taken from different cells - using the skills of genetic engineers.
A normal T cell has a special TCR receptor (T-cell receptor) . He "feels" all the cells of the body in his path and, if he senses some foreign molecule on the cell surface, sends an activating signal to the T-cell. She, in turn, either cracks down on an unwanted newcomer herself, or secretes special active substances (cytokines) and calls on other cells of the immune system to "sort things out". They kill T cells very effectively.
True, not very accurate. There are far fewer TCR species than antigens exist. Therefore, T cells are able to recognize many antigens by their TCR, but only approximately. Cancer cells often take advantage of this weakness in our security system and pretend to be "their own."
Evolution solved the problem as best it could: in the human body there is another mechanism for identifying outsiders: antibodies . These are special proteins that are secreted by another class of immune cells: B-lymphocytes. In B-cells, in contrast to T-cells, each “client” has an individual approach.
The antibody is a protein structure in the form of a letter Y. At both ends of this "fork" there are sites that bind to the antigen. These sites may change with each next generation of antibodies to fit snugly to the antigen — like picking puzzle pieces. When a foreign antigen is detected, B cells secrete billions of antibodies, among which there is a selection for the most accurate match for the antigen. As a result, reference antibodies are obtained, “trained” especially for very precise recognition of a specific “alien” antigen.

Antibody adapted to find a specific antigen
However, recognizing does not always mean disabling. With this, the complexity of antibodies - independently destroy the "enemy", they can not in all cases.
So, in 1989, an Israeli chemist and immunologist Zelig Ashhar came up with the idea of combining the deadly power of weak-sighted T-cells and the sniper aiming of antibodies. He singled out the end sections of protein-antibodies that are able to tightly bind to the antigen of certain cancer cells, and “transplanted” them into a T-cell — they replaced part of the TCR responsible for the recognition of antigens.
Subsequently, he began working together with his American colleague, Stephen Rosenberg, they managed to make the chimeric receptor more efficient design, at the same time sensitive and selective.

The difference between normal T-cells and CAR-T-cells
In vitro studies have shown good results. Then the scientists again treated the mice, then painstakingly transferred the technique to humans.
Over time, CAR-T therapy has led to a modern look.
- First, using gene-molecular testing, specific mutations in human tumor cells are determined for which antibodies can be “tuned”.
- Then they take their own T-cells from a person, change them using bioengineering methods, instead of TCR, “transplanting” CAR, which is tuned to the identified mutations.
- Then the modified CAR-T cells multiply in vitro and are injected back into the human body, where they successfully recognize and kill the cancer cells.
In the clinical trials started in 2010, encouraging results were obtained immediately: in the treatment of lymphoma, 12 out of 13 patients showed improvement, and 4 had remission. In the treatment of leukemia, remission occurred in 17 out of 33 people.
In 2018, Nature Medicine published an article by American oncologists, where it was reported that they had been observing a patient completely healthy for two years after CAR-T therapy. She was cured of metastatic breast cancer with metastases. This picture of her in a kayak is given at the beginning of the article: after treatment she returned to work and goes on hikes.
New Hope. Will immunotherapy be a panacea?
Like other cancer treatments, immunotherapy has its limitations. Despite the fact that in some cases patients give a very good response to therapy with inhibitors of immune control points, in 60% of cases either the acquired resistance develops or primary resistance to anti-PD-1 or anti-CTLA-4 drugs is observed: the tumor simply does not respond for treatment or quickly adapts and learns to "bypass" it.
In addition to PD-1, PD-L1 / 2, CTLA-4, CD28 and B7, there are a lot of other co-receptors on the surfaces of T-cells and tumor cells, the effect of which has not yet been studied as well as the work of control points, but they also affect on the immune response. One of the areas of work is the effect on these co-receptors.
In addition, ICT therapy is complemented by the introduction of vaccines, cytokines, beta-blockers - and this approach also works well in some cases .
CAR-T therapy is still extremely expensive and is still only entering the stage of commercial use: research is being carried out in scientific groups of Ashhar and Rosenberg, other researchers - each of the groups creates special types of CAR-T with directed action against a certain type of cancer. But for now this is only research, testing and testing. It will take several years before it turns into a spent mass treatment method - but even then it will not be possible to give 100% guarantees.
But while scientists are conducting research, doctors are introducing experimental treatment regimens using those achievements that already exist. And the most noticeable effect is given by the combination of immunotherapy with the classic "three pillars" of oncology: radiation and chemotherapy, surgery. When combining these methods, synergy is always obtained: together they work more efficiently than in turn.
, ( ) , , , : .
. , , . , , , , . . , 50% . , .
. 3 , 3 «», , , .
– . , , . , 2-3 . , , , . , .
. « 24/7» , , - . , . – . – . III-IV . , , .
, – . , , , . , , . , . .
Source: https://habr.com/ru/post/433130/
All Articles