Illusion of purity: does water mineralization affect its quality, and how will the TDS-meter help us?
In the 90s it was fashionable to buy nitrate level gauges. Food dyes, preservatives - is nonsense, but the watermelon for nitrates should be checked. Alas, this story was a profanation. But now from each youtube-iron they talk about water quality meters - TDS-meters. On the wave of general detox and the desire for a healthy lifestyle, many people want to get a magic wand, which will ensure a healthy lifestyle and eternal youth, indicating what to drink and what not to drink.
The temptation to determine the quality of water “here and now” by a pretty gadget, resembling an electronic thermometer, is very high. HYIP around TDS-meters continues to multiply, because they promise to replace the laboratory, calculate the dissolved impurities and decide, "to drink or not to drink?".
All this is an amazing scale substitution of concepts. After all, the definition of "purity" of water according to the content of unknown dissolved impurities can be put on a par with the measurement of a boa in parrots.
')

What is wrong in the history of TDS meters and drinking water standards, can you trust the TDS meter and drink the liquid “approved” by it - below we understand in detail and use frightening terms.
Let's start from afar: what is “clean and high-quality” water?
It is enough for someone that the tap water is clear and does not smell, someone freezes to give a “natural structure”, some are filtered, measuring cleanliness by the absence of scale, and an advanced user with a TDS meter writes a review on the topic “a bad filter, and the water from it is dirty ”, getting a high ppm value. Let's explain it, but first things first:
A word about standards: cleanliness is a loose concept
Since there is nothing absolutely pure in nature, then drinking water is a solution with impurities. Among them: conditionally useful, harmful, harmless and even "harmless, but unpleasant." The content of impurities in the aqueducts of the world is governed by national laws, sometimes based on the recommendations of WHO. The levels of permissible concentrations of substances, however, are not uniform.
The difference is due to the geological features of the countries and reasonable rationality. Under the conditions of a megalopolis, it is inappropriate to abandon steel pipes to remove rust, it is not economically viable to reduce stiffness, it is impossible to ensure bacteriological safety without chlorination (in most water mains). If high concentrations of toxic impurities are constantly present in the water due to geology in the territory or industry, local standards are “tailored” to the situation.
Consider the differences of standards on the example of such a harmful impurity as arsenic. Recommendation of the World Health Organization: the content of this element in drinking water should not exceed 0.01 mg / liter. Although it is better that it is not in the water at all, because a few years ago, arsenic was officially recognized as a carcinogen by WHO.
Back in the 1990s. In Bangladesh, the widespread presence of arsenic in well water was recorded. The national arsenic standard is now raised to 0.05 mg / liter. However, even today, tens of millions of people in the country are at risk of arsenic exposure in concentrations far in excess of 0.05 mg / liter.
WHO notes a similar natural anomaly in Argentina, Cambodia, Chile, China, Hungary, Mexico, Romania, Thailand, the United States and Vietnam. In particular, the authorities of Argentina, even after a heated debate and a five-year search for a solution, unfortunately did not find a way to ensure a reduction in the national arsenic standard from 0.05 mg / l to the WHO-recommended 0.01 mg / liter.
Russian SanPiN Standards for Arsenic versus WHO, EU and US Standards

But WHO is not always right. Some regions of the world suffer from excessive copper. The result of the active use of copper and its alloys in plumbing has become high national MPCs for copper in our country, in the USA (1 mg / l) and in Germany (2 mg / l). The WHO recommendation, however, is loyal and does not lower this bar, despite the fact that 1 and 2 mg / l is very, very much.
Standards of Russian SanPiN for copper in comparison with WHO, EU and US standards

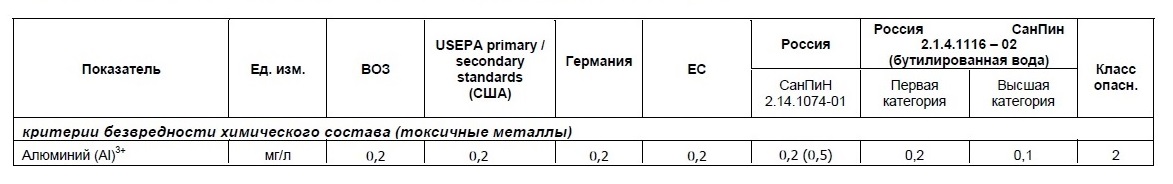
The situation is similar to aluminum. The recommendations are not very strict: aluminum salts are used for coagulation in the process of municipal water purification, therefore, the MPC excess is observed everywhere. And you can not refuse, and the presence is harmful. Over the past decade, the MPC for aluminum has decreased, but the actual numbers may seem wild to our grandchildren.
Standards of the Russian SanPiN for aluminum in comparison with the standards of WHO, EU and the USA
Sanitary standards are imperfect, constantly tightened, and you should not treat them as the ultimate truth. Just remember: lead, arsenic and aluminum do not become less toxic from what is present within the MPC.
Municipal preparation of water anywhere in the world does not have the task to apply “clean water” to the tap. This is justified by the fact that most of the water drains into the sewage system, bypassing our stomachs. Safe and reasonably cheap water is supplied to the water supply system, which will not be poisoned if you accidentally swallow it in the shower or drink from despair after a stormy party. Therefore, we keep in mind:
Or rather - to the impurities in drinking water. Some substances do not prevent it from remaining harmless, while deteriorating its organoleptic properties. So behave carbonates of calcium, magnesium, sodium chloride, phosphates, sulfates. True, they show their character when the concentrations are large enough.
Let it be bright, noisy, but harmless parrots .
Part of the substances - xenobiotics, poisons in any form and at any concentration. These are lead, mercury, chromium, arsenic, organochlorine compounds and many other substances. As we have already found out, their concentration in tap water is determined both by our abilities in purification and external factors. They are dangerous, they do not spoil life immediately, but they do it effectively, for example, by provoking the onset and development of cancerous tumors.
Let it be a quiet but dangerous boa .
As a company that manufactures filters, we constantly receive “claims” from buyers who evaluate the performance of the filter according to the rate of occurrence of scale. That is, the noticeable and bright organoleptic properties of water - hardness and mineralization - are often perceived as the main criterion for the quality of cleaning.

The main task of water filters is to protect against toxic cocktails from chlorine residues (chlorine is poison), its organic derivatives and wastes of industrial and agricultural enterprises: phenols, nitrates, pesticides, heavy metals, and so on. Depending on the model, filters can additionally protect against bacteria, viruses, allergens, antibiotics, and hundreds of other hidden threats.
Is mineralization a pollution and quality criterion?
Above, we discussed that there are no uniform international standards for impurities in drinking water. In this light, the measuring scales, which we see in numerous TDS videos, all the more seem like a colorful marketing abstraction. An example of a typical illustration:
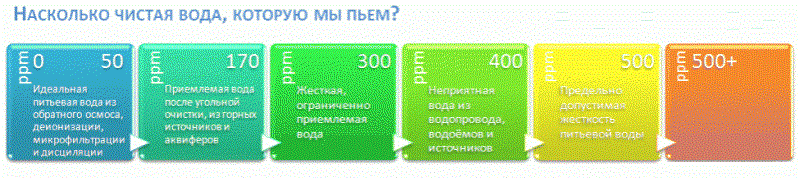
Similar illustrations travel from roller to roller, stating that samples marked “from 400” are no longer drinkable. It is curious that the author of a typical youtube test is not surprised by the figure of 4500 in a glass of highly useful mineral water from a respected Russian brand.
Mineralization is the physico-chemical parameter of an aqueous solution, the same as, for example, its temperature. Of course, even the temperature can be considered a parameter of water quality, when the holidays are short and the water in the sea is cool. Or when you really want to drink, but the water just boiled. With mineralization, too, everything is relative and depends on specific conditions.
The use of water of different mineralization is a matter of habit. The inhabitants of the chalk hills or those who grew up near the sea, where the groundwater is also salty (hello, Evpatoria!), Drink this water every day. SanPiN and WHO allow total mineralization (by dry residue) not higher than 1 g per liter (1000 ppm). There is no sacred meaning in the knowledge that the total mineralization of your water is 100 or 1000 units per TDS meter. From the point of view of domestic inconvenience, it is an unaesthetic sediment in a kettle, damage to expensive water-heating appliances (boiler), tasteless tea and dry skin. But this is obvious without a gadget.
Only the reverse osmosis filter is the most effective in the fight against dissolved impurities. The principle of its device differs from the flow filter due to the presence of a special membrane. It is she who separates tap water into purified and impurity concentrate, which is drained into the drainage.
A sorption (i.e. flow-through) water purifier cannot “remove” salts from water, including calcium or magnesium ions. The softening version of the flow filter has an ion-exchange module, which provides for the replacement of calcium and magnesium ions with sodium ions (more rarely, hydrogen), which do not precipitate during boiling.
The reasons may be many.
In particular, in the process of ion exchange, calcium and magnesium change to sodium. Calcium is a doubly charged ion, sodium is a single-charged one. So in place of one calcium goes two sodium ions.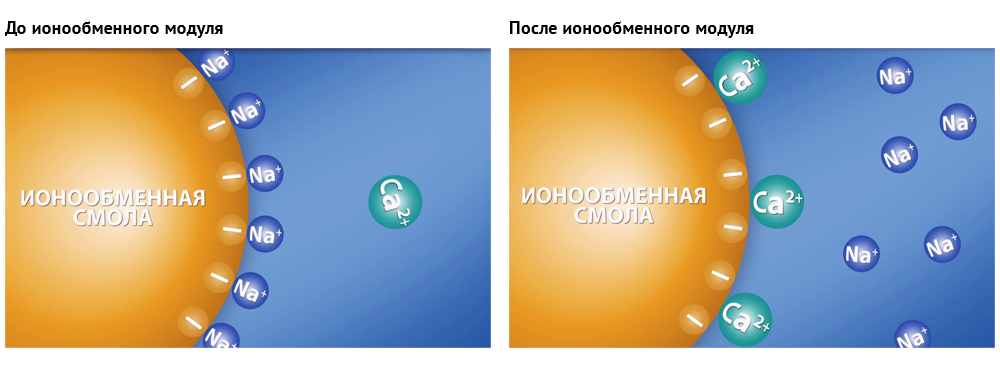
And:
Without quantitative and qualitative analysis of water before ion exchange, it is impossible to predict how the true TDS value and electrical conductivity will change. So, the idea to measure TDS after the sorption filter is meaningless.
The only true method for measuring TDS (total dissolved solids) is evaporation and weighing. And the fact that manufacturers are called TDS-meters, in fact - conductometers.
Extremely simplified circuit of the conductometer:
Water without impurities (pure):
A. has high resistance and low conductivity;
B. it has few ions (charge carriers);
C. when electrolytes get into it (clean water is salted), charge carriers are formed - ions that increase its electrical conductivity, as they are carriers of electric charge.
What is wrong with TDS-meters
Problem 1 - Terminology
Soluble non-electrolytes present in water will not add conductivity to water, i.e. A conductometric TDS meter immersed in sweet tea brewed in distilled water will show an extremely low value, but when evaporated and weighed, the dry residue will be high.
TDS (total dissolved solids) means the mass of the solid residue, which is obtained if all the water is evaporated. In the solid residue will remain both soluble electrolytes (salts, acids, bases), and soluble non-electrolytes, and insoluble solids (sand, clay), whose total mass is called TDS in chemistry. By the way, in the domestic terminology there is the term “total salt content”, which more accurately reflects the value that the conductivity meter measures.
Problem 2 - Measuring Equivalent
The TDS meter is most often graded on sodium chloride. Therefore, if the ionic composition of the test water differs from sodium chloride (very hard water containing calcium / magnesium ions and bicarbonate ions), then the estimates of salinity in ppm, calculated by sodium chloride calibration, are very approximate.
In drinking water, sodium chloride is rarely the dominant component. Macro cations contain ions of calcium, magnesium, potassium, etc. Anions are chloride, sulfate, carbonate / bicarbonate, silicate, phosphate, etc. All of them with different mobility carry an electric charge.
So, in the strict sense it is impossible to say about a TDS meter that it measures “water hardness”, “salt concentration” or, God forbid, “water pollution”. The only thing that can be said about him is that he gives on the display, expressed in ppm (mg / l), an equivalent concentration of sodium chloride solution with a temperature of 25 ° C, which will give the same amount of electrical conductivity that the device has recorded here and now.
What is the profit of ownership of the conductivity meter?
The video clearly explains which liquids, apart from the salt solution, give an exorbitant ppm, how water temperature affects the measurement accuracy, and also conduct an experiment with an insecticide that is not an electrolyte.
And in the “dry solid residue” on the topic of a TDS meter, we have:
1. With the help of a TDS meter alone you will not determine the purity and safety of tap water or any other solution, since:
2. The quality of the sorption water purifier cannot be determined with the TDS meter either, since this type of filter does not always change the mineral composition of the water.
1. TDS-meter will help to estimate the total mineralization of water in cases when there is a question about installing a filter, the principle of which is based on the change in the mineral composition of water (reverse osmosis).
2. The TDS-meter will help to understand when it is time to change the membrane in the reverse osmosis filter, or its resource is still sufficient. When replacing the membrane is a great way to check if it is defective.
3. And measuring different liquids with a TDS meter is a great way to spend time with children and a reason to tell them about what electrical conductivity is and why measuring a boa in parrots is fun, though not practical.
The temptation to determine the quality of water “here and now” by a pretty gadget, resembling an electronic thermometer, is very high. HYIP around TDS-meters continues to multiply, because they promise to replace the laboratory, calculate the dissolved impurities and decide, "to drink or not to drink?".
All this is an amazing scale substitution of concepts. After all, the definition of "purity" of water according to the content of unknown dissolved impurities can be put on a par with the measurement of a boa in parrots.
')

What is wrong in the history of TDS meters and drinking water standards, can you trust the TDS meter and drink the liquid “approved” by it - below we understand in detail and use frightening terms.
Let's start from afar: what is “clean and high-quality” water?
It is enough for someone that the tap water is clear and does not smell, someone freezes to give a “natural structure”, some are filtered, measuring cleanliness by the absence of scale, and an advanced user with a TDS meter writes a review on the topic “a bad filter, and the water from it is dirty ”, getting a high ppm value. Let's explain it, but first things first:
- on water treatment standards and the nuances of the concepts of "clean" - "drinking"
- is mineralization a pollution and quality criterion
- How does the TDS meter change after the filter?
- how the device is arranged, and why it is incorrect to call it a TDS meter
- what is the profit of the owner
A word about standards: cleanliness is a loose concept
Since there is nothing absolutely pure in nature, then drinking water is a solution with impurities. Among them: conditionally useful, harmful, harmless and even "harmless, but unpleasant." The content of impurities in the aqueducts of the world is governed by national laws, sometimes based on the recommendations of WHO. The levels of permissible concentrations of substances, however, are not uniform.
The difference is due to the geological features of the countries and reasonable rationality. Under the conditions of a megalopolis, it is inappropriate to abandon steel pipes to remove rust, it is not economically viable to reduce stiffness, it is impossible to ensure bacteriological safety without chlorination (in most water mains). If high concentrations of toxic impurities are constantly present in the water due to geology in the territory or industry, local standards are “tailored” to the situation.
What Argentinean - MPC, then the German - exceeding the norms of WHO
Consider the differences of standards on the example of such a harmful impurity as arsenic. Recommendation of the World Health Organization: the content of this element in drinking water should not exceed 0.01 mg / liter. Although it is better that it is not in the water at all, because a few years ago, arsenic was officially recognized as a carcinogen by WHO.
WHO: “High concentrations of arsenic are naturally present in groundwater in a number of countries . ”
Back in the 1990s. In Bangladesh, the widespread presence of arsenic in well water was recorded. The national arsenic standard is now raised to 0.05 mg / liter. However, even today, tens of millions of people in the country are at risk of arsenic exposure in concentrations far in excess of 0.05 mg / liter.
WHO notes a similar natural anomaly in Argentina, Cambodia, Chile, China, Hungary, Mexico, Romania, Thailand, the United States and Vietnam. In particular, the authorities of Argentina, even after a heated debate and a five-year search for a solution, unfortunately did not find a way to ensure a reduction in the national arsenic standard from 0.05 mg / l to the WHO-recommended 0.01 mg / liter.
Russian SanPiN Standards for Arsenic versus WHO, EU and US Standards

But WHO is not always right. Some regions of the world suffer from excessive copper. The result of the active use of copper and its alloys in plumbing has become high national MPCs for copper in our country, in the USA (1 mg / l) and in Germany (2 mg / l). The WHO recommendation, however, is loyal and does not lower this bar, despite the fact that 1 and 2 mg / l is very, very much.
Standards of Russian SanPiN for copper in comparison with WHO, EU and US standards

The situation is similar to aluminum. The recommendations are not very strict: aluminum salts are used for coagulation in the process of municipal water purification, therefore, the MPC excess is observed everywhere. And you can not refuse, and the presence is harmful. Over the past decade, the MPC for aluminum has decreased, but the actual numbers may seem wild to our grandchildren.
Standards of the Russian SanPiN for aluminum in comparison with the standards of WHO, EU and the USA

Sanitary standards are imperfect, constantly tightened, and you should not treat them as the ultimate truth. Just remember: lead, arsenic and aluminum do not become less toxic from what is present within the MPC.
Municipal preparation of water anywhere in the world does not have the task to apply “clean water” to the tap. This is justified by the fact that most of the water drains into the sewage system, bypassing our stomachs. Safe and reasonably cheap water is supplied to the water supply system, which will not be poisoned if you accidentally swallow it in the shower or drink from despair after a stormy party. Therefore, we keep in mind:
Water corresponding SanPiN - "drinking". However, putting thetest sample in the analyzerhand on the heart, not so clean for long use as a drinking. This is the first step in the "drinking water rating", below which there are liquids that are dangerous to drink without additional treatment.
Back to our parrots
Or rather - to the impurities in drinking water. Some substances do not prevent it from remaining harmless, while deteriorating its organoleptic properties. So behave carbonates of calcium, magnesium, sodium chloride, phosphates, sulfates. True, they show their character when the concentrations are large enough.
Let it be bright, noisy, but harmless parrots .
Part of the substances - xenobiotics, poisons in any form and at any concentration. These are lead, mercury, chromium, arsenic, organochlorine compounds and many other substances. As we have already found out, their concentration in tap water is determined both by our abilities in purification and external factors. They are dangerous, they do not spoil life immediately, but they do it effectively, for example, by provoking the onset and development of cancerous tumors.
Let it be a quiet but dangerous boa .
As a company that manufactures filters, we constantly receive “claims” from buyers who evaluate the performance of the filter according to the rate of occurrence of scale. That is, the noticeable and bright organoleptic properties of water - hardness and mineralization - are often perceived as the main criterion for the quality of cleaning.
The use of the TDS-meter will certainly help the “experimenter” to estimate the size of a pack of parrots and even understand that there are about 38 of them. However, he will not see a boa behind them.

The main task of water filters is to protect against toxic cocktails from chlorine residues (chlorine is poison), its organic derivatives and wastes of industrial and agricultural enterprises: phenols, nitrates, pesticides, heavy metals, and so on. Depending on the model, filters can additionally protect against bacteria, viruses, allergens, antibiotics, and hundreds of other hidden threats.
Is mineralization a pollution and quality criterion?
Above, we discussed that there are no uniform international standards for impurities in drinking water. In this light, the measuring scales, which we see in numerous TDS videos, all the more seem like a colorful marketing abstraction. An example of a typical illustration:

Similar illustrations travel from roller to roller, stating that samples marked “from 400” are no longer drinkable. It is curious that the author of a typical youtube test is not surprised by the figure of 4500 in a glass of highly useful mineral water from a respected Russian brand.
Mineralization is the physico-chemical parameter of an aqueous solution, the same as, for example, its temperature. Of course, even the temperature can be considered a parameter of water quality, when the holidays are short and the water in the sea is cool. Or when you really want to drink, but the water just boiled. With mineralization, too, everything is relative and depends on specific conditions.
Salinity is the same "criterion" of water quality as its temperature. This indicator for fresh water is not toxic and is not pollution.
The use of water of different mineralization is a matter of habit. The inhabitants of the chalk hills or those who grew up near the sea, where the groundwater is also salty (hello, Evpatoria!), Drink this water every day. SanPiN and WHO allow total mineralization (by dry residue) not higher than 1 g per liter (1000 ppm). There is no sacred meaning in the knowledge that the total mineralization of your water is 100 or 1000 units per TDS meter. From the point of view of domestic inconvenience, it is an unaesthetic sediment in a kettle, damage to expensive water-heating appliances (boiler), tasteless tea and dry skin. But this is obvious without a gadget.
Why the water quality after softening flow filters is meaningless to measure with a TDS meter
Only the reverse osmosis filter is the most effective in the fight against dissolved impurities. The principle of its device differs from the flow filter due to the presence of a special membrane. It is she who separates tap water into purified and impurity concentrate, which is drained into the drainage.
A sorption (i.e. flow-through) water purifier cannot “remove” salts from water, including calcium or magnesium ions. The softening version of the flow filter has an ion-exchange module, which provides for the replacement of calcium and magnesium ions with sodium ions (more rarely, hydrogen), which do not precipitate during boiling.
The change in the TDS meter readings after the passage of water through the ion exchange module of the flow filter is unpredictable. Some ions change to others, as the conductivity changes, it is very difficult to calculate. Oscillations occur both upwards and downwards.
The reasons may be many.
In particular, in the process of ion exchange, calcium and magnesium change to sodium. Calcium is a doubly charged ion, sodium is a single-charged one. So in place of one calcium goes two sodium ions.

And:
- not only chargeability is important, but also ion mobility, it is also different for elements;
- ion mobility and the rate of charge transfer strongly depend on the quality of the environment, for example, on which anions are around;
- impurity concentration is also important and affects the final change in electrical conductivity;
- even the temperature of water samples can affect the difference in readings, providing a difference of up to a hundred units.
Without quantitative and qualitative analysis of water before ion exchange, it is impossible to predict how the true TDS value and electrical conductivity will change. So, the idea to measure TDS after the sorption filter is meaningless.
The device and the true name of the TDS-meter
The only true method for measuring TDS (total dissolved solids) is evaporation and weighing. And the fact that manufacturers are called TDS-meters, in fact - conductometers.
Extremely simplified circuit of the conductometer:
- The two electrodes are separated by an insulating gap (air).
- A known potential (voltage) is applied to the electrodes.
- When a conductive medium (water with solutes) enters the gap between them, the amount of current that flows between the electrodes is measured.
Water without impurities (pure):
A. has high resistance and low conductivity;
B. it has few ions (charge carriers);
C. when electrolytes get into it (clean water is salted), charge carriers are formed - ions that increase its electrical conductivity, as they are carriers of electric charge.

What is wrong with TDS-meters
Problem 1 - Terminology
Soluble non-electrolytes present in water will not add conductivity to water, i.e. A conductometric TDS meter immersed in sweet tea brewed in distilled water will show an extremely low value, but when evaporated and weighed, the dry residue will be high.
TDS (total dissolved solids) means the mass of the solid residue, which is obtained if all the water is evaporated. In the solid residue will remain both soluble electrolytes (salts, acids, bases), and soluble non-electrolytes, and insoluble solids (sand, clay), whose total mass is called TDS in chemistry. By the way, in the domestic terminology there is the term “total salt content”, which more accurately reflects the value that the conductivity meter measures.
Problem 2 - Measuring Equivalent
The TDS meter is most often graded on sodium chloride. Therefore, if the ionic composition of the test water differs from sodium chloride (very hard water containing calcium / magnesium ions and bicarbonate ions), then the estimates of salinity in ppm, calculated by sodium chloride calibration, are very approximate.
In drinking water, sodium chloride is rarely the dominant component. Macro cations contain ions of calcium, magnesium, potassium, etc. Anions are chloride, sulfate, carbonate / bicarbonate, silicate, phosphate, etc. All of them with different mobility carry an electric charge.
So, in the strict sense it is impossible to say about a TDS meter that it measures “water hardness”, “salt concentration” or, God forbid, “water pollution”. The only thing that can be said about him is that he gives on the display, expressed in ppm (mg / l), an equivalent concentration of sodium chloride solution with a temperature of 25 ° C, which will give the same amount of electrical conductivity that the device has recorded here and now.
What is the profit of ownership of the conductivity meter?
The video clearly explains which liquids, apart from the salt solution, give an exorbitant ppm, how water temperature affects the measurement accuracy, and also conduct an experiment with an insecticide that is not an electrolyte.
And in the “dry solid residue” on the topic of a TDS meter, we have:
What a TDS meter can't do
1. With the help of a TDS meter alone you will not determine the purity and safety of tap water or any other solution, since:
- not all electrolyte substances are dangerous;
- Not all hazardous substances are electrolytes, and therefore remain invisible to the TDS meter.
2. The quality of the sorption water purifier cannot be determined with the TDS meter either, since this type of filter does not always change the mineral composition of the water.
When a TDS meter is useful
1. TDS-meter will help to estimate the total mineralization of water in cases when there is a question about installing a filter, the principle of which is based on the change in the mineral composition of water (reverse osmosis).
2. The TDS-meter will help to understand when it is time to change the membrane in the reverse osmosis filter, or its resource is still sufficient. When replacing the membrane is a great way to check if it is defective.
3. And measuring different liquids with a TDS meter is a great way to spend time with children and a reason to tell them about what electrical conductivity is and why measuring a boa in parrots is fun, though not practical.
Source: https://habr.com/ru/post/431050/
All Articles