Hydrogen energy: the beginning of a long journey

Earlier, we talked about how environmentally friendly the type of transport are electric buses. However, they did not mention one important point: with the increase in the number of electric transport, cities will need more electricity, which they often receive in environmentally unsafe ways. Fortunately, today the world has learned to receive energy with the help of wind, sun and even hydrogen. We decided to devote the new material to the latest source and tell about the peculiarities of hydrogen energy.
At first glance, hydrogen is an ideal fuel. Firstly, it is the most common element in the universe, and secondly, when it is burned, a large amount of energy is released and water is formed without releasing any harmful gases. The humankind has realized the advantages of hydrogen energy for a long time, but it is not in a hurry to use it on a large industrial scale.
Hydrogen fuel cells
The first hydrogen fuel cell was designed by the English scientist William Grove in the 30s of the XIX century. Grove tried to precipitate copper from an aqueous solution of copper sulfate on the iron surface and noticed that under the action of an electric current, water disintegrates into hydrogen and oxygen. After this discovery, Grove and Christian Schönbein, who worked in parallel with him, demonstrated the possibility of producing energy in a hydrogen-oxygen fuel cell using acid electrolyte.
')
Later, in 1959, Francis T. Bacon from Cambridge added an ion-exchange membrane to a hydrogen fuel cell to facilitate the transport of hydroxide ions. The US government and NASA immediately became interested in the invention of Bacon, the renewed fuel cell began to be used on the Apollo spacecraft as the main source of energy during their flights.

Hydrogen fuel cell from the Apollo service module, which produces electricity, heat and water for astronauts. Source: James Humphreys / Wikimedia Commons
Now the hydrogen fuel cell resembles a traditional galvanic cell with only one difference: the substance for the reaction is not stored in the cell, but is constantly supplied from the outside. As it seeps through the porous anode, hydrogen loses electrons, which go into an electrical circuit, and hydrogen cations pass through the membrane. Then, at the cathode, oxygen catches a proton and an external electron, as a result of which water is formed.
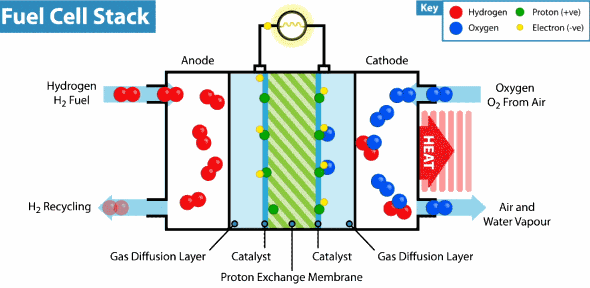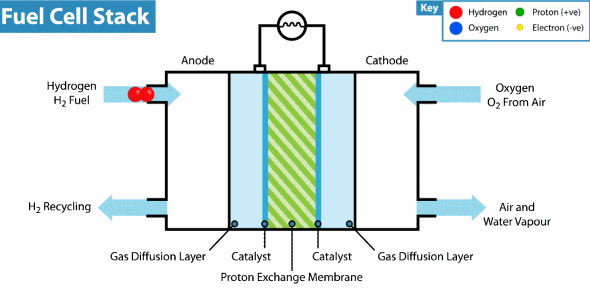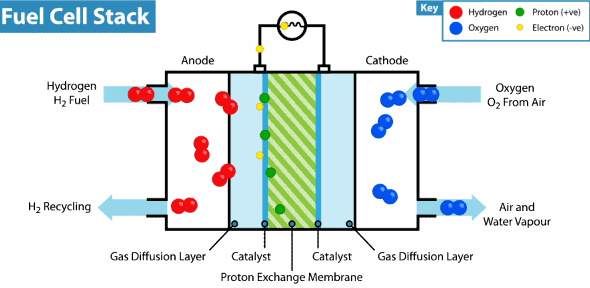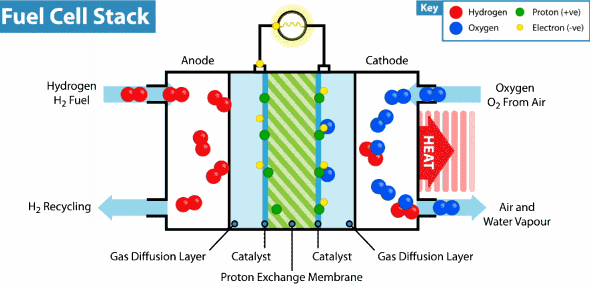
The principle of operation of the hydrogen fuel cell. Source: Geek.com
A voltage of about 0.7 V is removed from one fuel cell, so cells are combined into massive fuel cells with an acceptable output voltage and current. The theoretical voltage from a hydrogen cell can reach 1.23 V, but some of the energy goes into heat .
From the point of view of "green" energy, hydrogen fuel cells have extremely high efficiency - 60%. For comparison: The efficiency of the best internal combustion engines is 35-40%. For solar power plants, the coefficient is only 15-20%, but it depends strongly on weather conditions. The efficiency of the best wing wind power plants reaches 40%, which is comparable to steam generators, but wind turbines also require suitable weather conditions and expensive maintenance.
As we see, according to this parameter, hydrogen energy is the most attractive source of energy, but still there are a number of problems that prevent its mass use. The most important of them is the process of hydrogen production.
Mining problems
Hydrogen energy is environmentally friendly, but not autonomous. The fuel cell needs hydrogen, which is not found on Earth in its pure form. Hydrogen needs to be obtained, but all existing methods are either very costly or ineffective.
The most efficient in terms of the volume of hydrogen produced per unit of energy expended is the method of steam reforming of natural gas . Methane is combined with water vapor at a pressure of 2 MPa (about 19 atmospheres, i.e. pressure at a depth of about 190 m) and a temperature of about 800 degrees, resulting in a converted gas with a hydrogen content of 55-75%. Huge installations are needed for steam reforming, which can only be applied in production.

The tube furnace for steam reforming of methane is not the most ergonomic way of hydrogen production. Source: CTC-Euro
A more convenient and simple method is water electrolysis. With the passage of electric current through the treated water, a series of electrochemical reactions occur, resulting in the formation of hydrogen. A significant disadvantage of this method is the large energy consumption required for the reaction. That is, it turns out to be a somewhat strange situation: to obtain hydrogen energy, we need ... energy. In order to avoid unnecessary costs during electrolysis and the preservation of valuable resources, some companies are striving to develop a full-cycle “electricity-hydrogen-electricity” system in which energy production becomes possible without external recharge. An example of such a system is the development of Toshiba H2One.
Mobile power Toshiba H2One
We have developed a mobile mini-power station H2One, which converts water into hydrogen, and hydrogen into energy. To maintain electrolysis, it uses solar cells, and the excess energy is accumulated in batteries and ensures the operation of the system in the absence of sunlight. The resulting hydrogen is either directly fed to the fuel cells, or sent to storage in the built-in tank. In an hour, the H2One electrolyzer generates up to 2 m 3 of hydrogen, and the output provides power up to 55 kW. For the production of 1 m 3 of hydrogen, the station requires up to 2.5 m 3 of water.
For the time being, H2One station is not able to provide electricity for a large enterprise or a whole city, but for the functioning of small areas or organizations its energy will be quite enough. Due to its mobility, it can be used as well as a temporary solution in the conditions of natural disasters or emergency power cuts. In addition, unlike a diesel generator, which requires fuel for normal operation, a hydrogen power station only needs water.
Now Toshiba H2One is used in only a few cities in Japan - for example, it supplies electricity and hot water to a railway station in the city of Kawasaki.
Installation of H2One system in Kawasaki
Hydrogen future
Now hydrogen fuel cells provide energy and portable power banks, city buses with cars, and rail transport (we’ll talk more about hydrogen in the automotive industry in our next post). Unexpectedly, hydrogen fuel cells proved to be an excellent solution for quadcopters - with a mass similar to a battery, hydrogen stores up to five times the flight time. At the same time frost does not affect the efficiency. Experimental fuel cell drones manufactured by the Russian company AT Energy were used for filming at the Sochi Olympics.
It became known that in the upcoming Olympics in Tokyo, hydrogen will be used in cars, in the production of electricity and heat, and will also become the main source of energy for the Olympic village. To do this, commissioned by Toshiba Energy Systems & Solutions Corp. One of the world's largest hydrogen production stations is under construction in the Japanese city of Namie. The station will consume up to 10 MW of energy obtained from "green" sources, generating by electrolysis up to 900 tons of hydrogen per year.
Hydrogen energy is our “reserve for the future” when fossil fuels will have to be completely abandoned and renewable energy sources will not be able to meet the needs of humanity. According to the forecast of Markets & Markets, the volume of global hydrogen production, which now amounts to $ 115 billion, will increase to $ 154 billion by 2022. But in the near future, mass introduction of the technology is unlikely to occur, it is necessary to solve a number of problems associated with the production and operation of special power plants and reduce their cost. . When technological barriers are overcome, hydrogen energy will reach a new level and, perhaps, will be just as common as traditional or hydropower today.
Source: https://habr.com/ru/post/428511/
All Articles