War on Disease: Revising Old Views
Despite the continuous flow of discoveries in the field of medicine, some diseases are still not amenable to researchers. Scientists are looking for fresh ideas in already well-studied areas.

The cage is tiny and huge at the same time.
As scientists penetrate deeper into the mechanisms that underlie difficult to cure diseases (such as diabetes or Alzheimer's disease), they are increasingly approaching the limits of scientific knowledge, reaching the darkest nooks of science in search of answers.
However, the answers to complex questions are not always obvious, even if we consider them from a different angle, so it is worth occasionally returning to the known and reviewing familiar facts.
')
For example, recently, a new body was “opened”, hiding “in plain sight.”
Interstitium - a system of cavities filled with fluid. Now it is believed that this is one of the largest organs of the body.
Previously, the interstitium was considered something unimportant — something like a glue to support “real” organs that perform important functions. However, when, thanks to advanced imaging technology, it was possible to look closely at it - its size and importance became apparent.
Scientists are wondering if the new organ could not clarify the cause of the unpleasant ability of edema, fibrosis and cancer to spread rapidly.
It is well known that in search of discoveries, we may need to test every hypothesis — look under each stone. Interstitium teaches us that some "stones" need to be turned over and over at regular intervals.
In this article, we will look at known aspects of cell biology, try to rethink them and provide unusual ways to understand diseases.
The cytoskeleton is a complex network of proteins in the cytoplasm of each cell. The term was first used by Nikolai Konstantinovich Koltsov in 1903. One of the main components of the cytoskeleton are long tubular proteins called microtubules.
Microtubules not only help maintain cell structure, but also play a crucial role in cell division and transfer of compounds around the cytoplasm. Microtubule dysfunction is associated with neurodegenerative conditions, including those known as Parkinson's and Alzheimer's.
Neurofibrillary glomeruli, which are abnormally twisted threads of tau protein, are one of the hallmarks of Alzheimer's disease. Usually, in combination with phosphate molecules, tau protein helps stabilize microtubules. However, in Alzheimer's neurons, tau proteins carry four times more phosphate than usual.
Hyperphosphorylation reduces the stability of microtubules, the speed of their creation, and can also lead to their destruction.
How exactly the change in microtubule production leads to neurodegeneration is not fully understood, however, researchers hope that intervening in these processes will one day help to treat or prevent Alzheimer's disease.
Microtubule problems are not associated exclusively with neurological conditions. Since the 1990s, scientists have been debating whether they could be the cause of cellular changes leading to a heart attack. The latest study on this issue concluded that the chemical changes in the microtubule network of cardiac cells made them more rigid and less able to contract as they should.
The authors of the study believe that the development of drugs that target microtubules may eventually become a viable way to "improve cardiac function."
If you studied mitochondria in a school biology course, most likely you only remember that "mitochondria are cell power plants." Nowadays, scientists are wondering if the mitochondria, which were discovered as early as the 1800s, cannot be associated with a number of diseases.

Mitochondria is more than just a powerhouse.
The role of mitochondria in the development of Parkinson's disease has received the most attention.
Over the years, various failures in their work have been implied as causes of Parkinson's disease. For example, malfunctions can occur in complex chemical pathways of energy generation in mitochondria. Another problem is mutations in mitochondrial DNA.
Mitochondria can be damaged due to the accumulation of reactive oxygen species, which are produced as a by-product of energy production. And yet, how do these failures lead to pronounced symptoms of Parkinson's disease? In the end, there are mitochondria in almost every cell of the human body.
The answer seems to lie in the type of cells affected by Parkinson's disease: dopaminergic neurons. These cells are very susceptible to mitochondrial dysfunction. This is partly due to the fact that they are particularly sensitive to oxidative stress. Dopaminergic neurons are also significantly dependent on calcium, an element whose level is controlled by mitochondria. Without mitochondrial control, dopaminergic nerve cells suffer disproportionately.
The role of mitochondria in the development of cancer is also discussed. Malignant cells uncontrollably divide and multiply - it is energy-intensive, and therefore, the main suspect is mitochondria.
In addition to the ability of mitochondria to generate energy for cancer cells, they also help cells adapt to new or stressful conditions. Since cancer cells have an uncanny ability to move from one part of the body to another, settle in a new place and continue to multiply tirelessly, mitochondria and here are the main suspect.
In addition to Parkinson's disease and cancer, there is evidence that mitochondria are associated with non-alcoholic fatty liver disease and certain lung diseases. We still have a lot to learn about how these hardworking organelles affect the development of diseases.
Bacteriophages are viruses attacking the bacteria. Not surprisingly, with increasing interest in intestinal bacteria, they began to pay attention to bacteriophages. After all, if bacteria can affect health, it means that killing them, of course, also affects it.
Bacteria are present in all ecosystems on Earth. Their number is difficult to estimate. Bacteriophages, however, outnumber them; one author calls them "almost ubiquitous."

Bacteriophage - adding complexity to an already complex
The effect of the microbiome on health is an intricate network of interactions that we are just beginning to unravel. If we add to this virus (a set of resident viruses in the human body), then the complexity of the task increases exponentially.
We already know how big the role of bacteria is in diseases and for a healthy state of the body. From here, only a small step is required to understand how useful bacteriophages (specific to different strains of bacteria) can become useful for medicine.
In fact, bacteriophages were already used to treat infections in the 1920s and 30s. However, with the advent of antibiotics, which are easier and cheaper to store and produce, interest in bacteriophages has fallen. However, due to the danger of bacterial resistance to antibiotics, it is quite possible to return to treatment with bacteriophages.
In addition, bacteriophages have an important advantage - they can be specific to a single strain of bacteria, unlike antibiotics, which directly affect a wide range of bacteria.
Although a revival of interest in bacteriophages has just emerged, some researchers already see their potential applicability in the fight against cardiovascular and autoimmune diseases, graft rejection and cancer.
Each cell is covered with a lipid membrane that allows one chemical to enter and exit, while others do not. Thus, lipid membranes are not just shells - they are complex protein complexes.
Lipid rafts are separate islands in a membrane complex. They contain channels and other structures. The exact purpose of these structures causes heated debate. Scientists are trying hard to figure out what they can mean for a number of conditions, including depression.
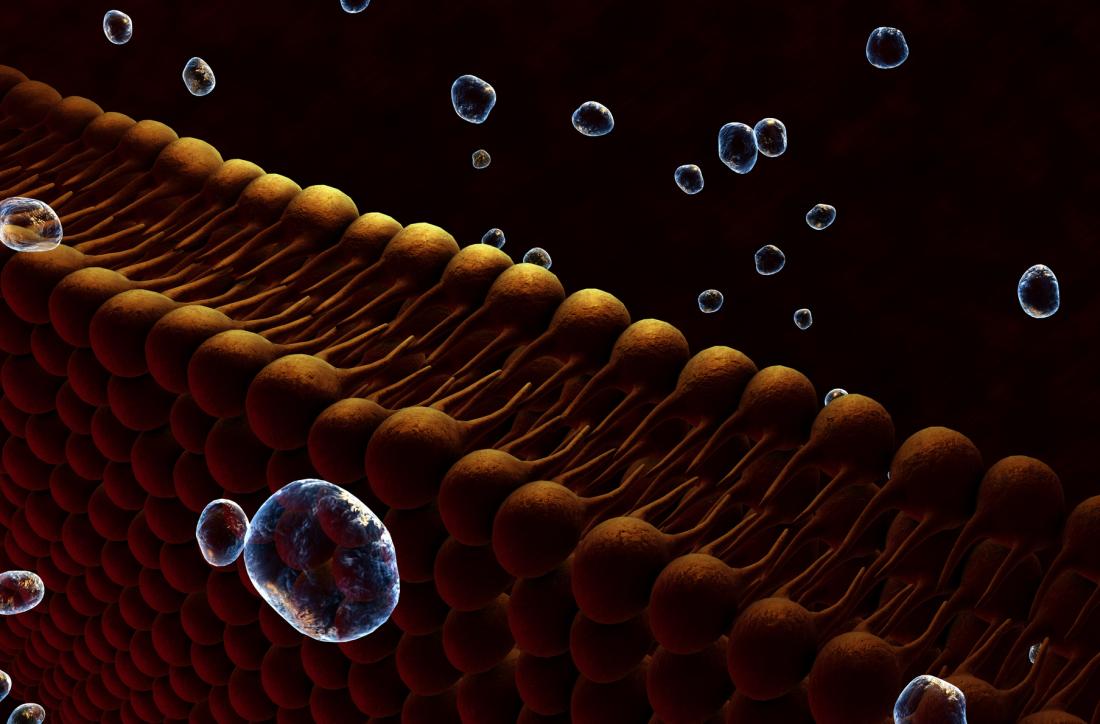
Lipid membrane - much more than just a shell.
Recent studies have shown that understanding the workings of these regions can help us figure out how antidepressants work.
G-proteins are signal-transmitting protein switches. They are deactivated when drifting into lipid rafts. On the one hand, when the activity of g-proteins decreases, the transmission of signals through neurons also decreases, which, theoretically, can cause some symptoms of depression. On the other hand, antidepressants have been shown to displace g-proteins from lipid rafts, thereby reducing the symptoms of depression.
There are studies that have examined the potential role of lipid rafts in drug resistance, metastasis in pancreatic and ovarian cancer, as well as cognitive decline in Alzheimer's disease.
The bilayer structure of the lipid membrane was first discovered in the middle of the last century, however, lipid rafts are a relatively new discovery. Many questions about their structure and function are still unanswered.
Extracellular vesicles are tiny sacs that transport chemicals between cells. They serve to connect cells and play a role in processes such as coagulation, cell aging and the immune response.
Since they convey messages to and fro, it is not surprising that something can break, which means that vesicles can potentially be associated with diseases.
In addition, since they can carry complex molecules, including proteins and DNA, there is every chance that they can transport disease-specific materials, such as proteins involved in neurodegenerative diseases.
Cancers also produce extracellular vesicles, and although their role is not fully understood, it is likely that they help the cancer cells to settle in remote places.
If we learn to decipher these intercellular signals, we can get an idea of the many processes associated with diseases. In theory, all we have to do is crack the code. However, this does not cancel the monumentality of the task.
If you recall a course in biology, then maybe you have a dim memory of a strange Latin term - the endoplasmic reticulum (ER). If you're lucky, you might even remember that this is an interconnected network of oblate cavities inside the cytoplasm, located close to the nucleus. The ER was first detected under a microscope at the end of the 19th century. He is engaged in the folding of proteins, and also prepares them for the harsh living conditions outside the cell.
It is important that protein coagulation occurs correctly; if this is not the case, the ER will not transmit them to the final destination. During stress, when the ER works more intensely, incorrectly folded proteins can form. This triggers a reaction called unfolded protein response (UPR).
The UPR attempts to bring the cells back to normal functioning. It cleans the cell from unfolded proteins. To achieve this, further protein production is stopped, poorly folded proteins are destroyed, and molecular mechanisms are activated that help interrupt incorrect folding.
If the ER does not have time to return the cell to normal functioning, and the UPR cannot bring the protein situation under control, then the cell is destroyed by apoptosis - a kind of cell suicide. ER stress and subsequent UPR are involved in a number of diseases, one of which is diabetes.
Insulin is produced by the beta cells of the pancreas, and since the level of this hormone changes throughout the day, ER-stress increases and decreases with it. This means that pancreatic cells are very dependent on the mechanism of UPR.
Studies have shown that high blood sugar has a stressful effect on protein synthesis. If the UPR cannot cope with the task, the pancreatic beta cells become dysfunctional and are destroyed by apoptosis. With the depletion of beta cells, insulin can no longer be produced when it is needed - diabetes develops.
Our days are exciting times for those involved in biomedicine, and as you can see from this brief review, we still have a lot to learn, and a retrospective of what has already been studied can be as useful as achieving new horizons.
Source: Tim Newman. The war on disease: Revisiting old haunts. Translated by Kostya Sviridov

The cage is tiny and huge at the same time.
As scientists penetrate deeper into the mechanisms that underlie difficult to cure diseases (such as diabetes or Alzheimer's disease), they are increasingly approaching the limits of scientific knowledge, reaching the darkest nooks of science in search of answers.
However, the answers to complex questions are not always obvious, even if we consider them from a different angle, so it is worth occasionally returning to the known and reviewing familiar facts.
')
For example, recently, a new body was “opened”, hiding “in plain sight.”
Interstitium - a system of cavities filled with fluid. Now it is believed that this is one of the largest organs of the body.
Previously, the interstitium was considered something unimportant — something like a glue to support “real” organs that perform important functions. However, when, thanks to advanced imaging technology, it was possible to look closely at it - its size and importance became apparent.
Scientists are wondering if the new organ could not clarify the cause of the unpleasant ability of edema, fibrosis and cancer to spread rapidly.
It is well known that in search of discoveries, we may need to test every hypothesis — look under each stone. Interstitium teaches us that some "stones" need to be turned over and over at regular intervals.
In this article, we will look at known aspects of cell biology, try to rethink them and provide unusual ways to understand diseases.
Microtubules: more than cell frame
The cytoskeleton is a complex network of proteins in the cytoplasm of each cell. The term was first used by Nikolai Konstantinovich Koltsov in 1903. One of the main components of the cytoskeleton are long tubular proteins called microtubules.
Microtubules not only help maintain cell structure, but also play a crucial role in cell division and transfer of compounds around the cytoplasm. Microtubule dysfunction is associated with neurodegenerative conditions, including those known as Parkinson's and Alzheimer's.
Neurofibrillary glomeruli, which are abnormally twisted threads of tau protein, are one of the hallmarks of Alzheimer's disease. Usually, in combination with phosphate molecules, tau protein helps stabilize microtubules. However, in Alzheimer's neurons, tau proteins carry four times more phosphate than usual.
Hyperphosphorylation reduces the stability of microtubules, the speed of their creation, and can also lead to their destruction.
How exactly the change in microtubule production leads to neurodegeneration is not fully understood, however, researchers hope that intervening in these processes will one day help to treat or prevent Alzheimer's disease.
Microtubule problems are not associated exclusively with neurological conditions. Since the 1990s, scientists have been debating whether they could be the cause of cellular changes leading to a heart attack. The latest study on this issue concluded that the chemical changes in the microtubule network of cardiac cells made them more rigid and less able to contract as they should.
The authors of the study believe that the development of drugs that target microtubules may eventually become a viable way to "improve cardiac function."
Not just power plants
If you studied mitochondria in a school biology course, most likely you only remember that "mitochondria are cell power plants." Nowadays, scientists are wondering if the mitochondria, which were discovered as early as the 1800s, cannot be associated with a number of diseases.

Mitochondria is more than just a powerhouse.
The role of mitochondria in the development of Parkinson's disease has received the most attention.
Over the years, various failures in their work have been implied as causes of Parkinson's disease. For example, malfunctions can occur in complex chemical pathways of energy generation in mitochondria. Another problem is mutations in mitochondrial DNA.
Mitochondria can be damaged due to the accumulation of reactive oxygen species, which are produced as a by-product of energy production. And yet, how do these failures lead to pronounced symptoms of Parkinson's disease? In the end, there are mitochondria in almost every cell of the human body.
The answer seems to lie in the type of cells affected by Parkinson's disease: dopaminergic neurons. These cells are very susceptible to mitochondrial dysfunction. This is partly due to the fact that they are particularly sensitive to oxidative stress. Dopaminergic neurons are also significantly dependent on calcium, an element whose level is controlled by mitochondria. Without mitochondrial control, dopaminergic nerve cells suffer disproportionately.
The role of mitochondria in the development of cancer is also discussed. Malignant cells uncontrollably divide and multiply - it is energy-intensive, and therefore, the main suspect is mitochondria.
In addition to the ability of mitochondria to generate energy for cancer cells, they also help cells adapt to new or stressful conditions. Since cancer cells have an uncanny ability to move from one part of the body to another, settle in a new place and continue to multiply tirelessly, mitochondria and here are the main suspect.
In addition to Parkinson's disease and cancer, there is evidence that mitochondria are associated with non-alcoholic fatty liver disease and certain lung diseases. We still have a lot to learn about how these hardworking organelles affect the development of diseases.
Microbiome - next level
Bacteriophages are viruses attacking the bacteria. Not surprisingly, with increasing interest in intestinal bacteria, they began to pay attention to bacteriophages. After all, if bacteria can affect health, it means that killing them, of course, also affects it.
Bacteria are present in all ecosystems on Earth. Their number is difficult to estimate. Bacteriophages, however, outnumber them; one author calls them "almost ubiquitous."

Bacteriophage - adding complexity to an already complex
The effect of the microbiome on health is an intricate network of interactions that we are just beginning to unravel. If we add to this virus (a set of resident viruses in the human body), then the complexity of the task increases exponentially.
We already know how big the role of bacteria is in diseases and for a healthy state of the body. From here, only a small step is required to understand how useful bacteriophages (specific to different strains of bacteria) can become useful for medicine.
In fact, bacteriophages were already used to treat infections in the 1920s and 30s. However, with the advent of antibiotics, which are easier and cheaper to store and produce, interest in bacteriophages has fallen. However, due to the danger of bacterial resistance to antibiotics, it is quite possible to return to treatment with bacteriophages.
In addition, bacteriophages have an important advantage - they can be specific to a single strain of bacteria, unlike antibiotics, which directly affect a wide range of bacteria.
Although a revival of interest in bacteriophages has just emerged, some researchers already see their potential applicability in the fight against cardiovascular and autoimmune diseases, graft rejection and cancer.
Setting sail on lipid rafts
Each cell is covered with a lipid membrane that allows one chemical to enter and exit, while others do not. Thus, lipid membranes are not just shells - they are complex protein complexes.
Lipid rafts are separate islands in a membrane complex. They contain channels and other structures. The exact purpose of these structures causes heated debate. Scientists are trying hard to figure out what they can mean for a number of conditions, including depression.

Lipid membrane - much more than just a shell.
Recent studies have shown that understanding the workings of these regions can help us figure out how antidepressants work.
G-proteins are signal-transmitting protein switches. They are deactivated when drifting into lipid rafts. On the one hand, when the activity of g-proteins decreases, the transmission of signals through neurons also decreases, which, theoretically, can cause some symptoms of depression. On the other hand, antidepressants have been shown to displace g-proteins from lipid rafts, thereby reducing the symptoms of depression.
There are studies that have examined the potential role of lipid rafts in drug resistance, metastasis in pancreatic and ovarian cancer, as well as cognitive decline in Alzheimer's disease.
The bilayer structure of the lipid membrane was first discovered in the middle of the last century, however, lipid rafts are a relatively new discovery. Many questions about their structure and function are still unanswered.
Good in small packages
Extracellular vesicles are tiny sacs that transport chemicals between cells. They serve to connect cells and play a role in processes such as coagulation, cell aging and the immune response.
Since they convey messages to and fro, it is not surprising that something can break, which means that vesicles can potentially be associated with diseases.
In addition, since they can carry complex molecules, including proteins and DNA, there is every chance that they can transport disease-specific materials, such as proteins involved in neurodegenerative diseases.
Cancers also produce extracellular vesicles, and although their role is not fully understood, it is likely that they help the cancer cells to settle in remote places.
If we learn to decipher these intercellular signals, we can get an idea of the many processes associated with diseases. In theory, all we have to do is crack the code. However, this does not cancel the monumentality of the task.
Something more than just folding
If you recall a course in biology, then maybe you have a dim memory of a strange Latin term - the endoplasmic reticulum (ER). If you're lucky, you might even remember that this is an interconnected network of oblate cavities inside the cytoplasm, located close to the nucleus. The ER was first detected under a microscope at the end of the 19th century. He is engaged in the folding of proteins, and also prepares them for the harsh living conditions outside the cell.
It is important that protein coagulation occurs correctly; if this is not the case, the ER will not transmit them to the final destination. During stress, when the ER works more intensely, incorrectly folded proteins can form. This triggers a reaction called unfolded protein response (UPR).
The UPR attempts to bring the cells back to normal functioning. It cleans the cell from unfolded proteins. To achieve this, further protein production is stopped, poorly folded proteins are destroyed, and molecular mechanisms are activated that help interrupt incorrect folding.
If the ER does not have time to return the cell to normal functioning, and the UPR cannot bring the protein situation under control, then the cell is destroyed by apoptosis - a kind of cell suicide. ER stress and subsequent UPR are involved in a number of diseases, one of which is diabetes.
Insulin is produced by the beta cells of the pancreas, and since the level of this hormone changes throughout the day, ER-stress increases and decreases with it. This means that pancreatic cells are very dependent on the mechanism of UPR.
Studies have shown that high blood sugar has a stressful effect on protein synthesis. If the UPR cannot cope with the task, the pancreatic beta cells become dysfunctional and are destroyed by apoptosis. With the depletion of beta cells, insulin can no longer be produced when it is needed - diabetes develops.
Our days are exciting times for those involved in biomedicine, and as you can see from this brief review, we still have a lot to learn, and a retrospective of what has already been studied can be as useful as achieving new horizons.
Source: Tim Newman. The war on disease: Revisiting old haunts. Translated by Kostya Sviridov
Source: https://habr.com/ru/post/422653/
All Articles