Five discoveries of fundamental physics, which were completely unexpected

Hubble Extreme Deep Field is our most detailed snapshot of the Universe, showing the galaxies that existed at a time when the age of the Universe was 3-4% of the present. The fact that we were able to see so much just by studying the seemingly black part of the sky for quite a long time was also an incredible surprise - but it did not make it to the list.
Studying the method of scientific knowledge, we imagine a clear procedure, following which, you can get to understand the natural processes occurring in the Universe. We start with an idea, carry out an experiment, and either confirm or refute it, depending on the result. That's just the real world is much more messy. Sometimes you can conduct an experiment and get a result that is radically different from expectations. Sometimes a correct explanation requires going beyond the limits of rational and logical conclusions. Today we have a good understanding of the Universe, but on the way to this we met many surprises. While making further progress, we will surely stumble upon something else. Here is a historical excursion describing the five greatest surprises in the history of science.

If the cannon is fired from the cannon in the direction opposite to the movement of the car, and at exactly the same speed, as a result, the velocity of the projectile will be zero. If we shot a light, it would always move at the speed of light.
1) The speed of light does not change due to the speed of the source. Imagine that you threw the ball very hard. Depending on the sport you are fond of, it can reach speeds of up to 45 m / s. Now imagine that you are in a train moving at a speed of 135 m / s. If you throw the ball from the train in the direction of its movement, how fast will it fly? Just add the speed - 180 m / s. Now imagine that instead of a ball you emit a ray of light. Add the speed of light and the speed of the train - and get the wrong answer.
')

The Michelson interferometer (above) showed a negligible change in the behavior of light (below, solid) compared to what it would be if Galileo’s law of relativity (below, dotted line) worked. The speed of light remained constant regardless of the direction of orientation of the interferometer — including the direction parallel to or perpendicular to the motion of the Earth in space.
This idea was central to Einstein’s special theory of relativity, but it was not Einstein who discovered it experimentally; it was Albert Michelson , whose advanced work demonstrated this result in the 1880s. Whether you start a beam of light in the direction of the Earth’s motion, perpendicular to this direction, or in the opposite direction - there is no difference. Light always moves at the same speed: c, the speed of light in a vacuum. Michelson developed an interferometer to measure the speed of the Earth relative to the ether , and instead paved the way for relativity. His 1907 Nobel Prize remains the most famous zero result and the most important in the history of science.

Helium atom with a nucleus at an approximate scale
2) 99.99% of the mass of the atom is concentrated in an incredibly dense core. Have you heard of the " pudding model of the atom "? Today, it seems strange, but at the beginning of the 20th century, it was generally accepted that an atom consists of a mixture of negatively charged electrons (raisins) embedded in a positively charged substance (pudding) that fills the entire space. Electrons can be removed from it, which explains the phenomenon of static electricity. For years, the composite model of the Thomson atom, with small electrons located on a positively charged substrate, was generally accepted. Until it was decided to check Ernest Rutherford.

Rutherford's experience with gold foil showed that the atom is mostly empty, but at one point there is a concentration of mass that is seriously greater than the mass of the alpha particle: the atomic nucleus.
Launching high-energy charged particles (from radioactive decay) into a very thin sheet of gold foil, Rutherford expected them to pass through it. Most of them did just that, but some bounced off spectacularly! As Rutherford recalls:
It was the most incredible thing that happened to me in life. It was almost as unbelievable as if you had fired a fifteen-inch shell at a napkin, and he would have bounced off of it and hit you.
Rutherford discovered an atomic nucleus containing almost the entire mass of an atom and limited to a volume of 10 -15 of the size of the entire atom. Thus was born modern physics, paving the way for the quantum revolution of the 20th century.

Two types (radiating and non-radiating) beta decay of a neutron . Beta decay, unlike alpha or gamma decay, does not conserve energy - if you cannot detect a neutrino.
3) "Missing energy" led to the discovery of a tiny, almost invisible particle. In all observed interactions between particles, energy is always conserved. It can be transformed from one type to another - potential, kinetic, rest mass, chemical, atomic, electric, etc. - but it cannot be created or destroyed. Therefore, almost a hundred years ago, it was so surprising to learn that some products of radioactive decay produce slightly less total energy than the original reagents. This led Bohr to the idea that energy is always saved ... except when it is lost. But Bohr was wrong, and Pauli had another idea.

The transformation of a neutron into a proton, an electron and an anti-electron neutrino - the solution of the problem of energy nonconservation during beta decay
Pauli argued that energy should be conserved, so in the 1930s he assumed the existence of a new particle: the neutrino. This "small neutron" particle did not enter into magnetic interactions, but instead possessed a tiny mass and carried with it kinetic energy. Many were skeptical of this, but in experiments among nuclear reaction products in the 1950s and 1960s, neutrinos and antineutrinos were eventually found, which helped lead physicists to the Standard Model and the model of weak nuclear interactions. This is a prime example of how theoretical predictions can sometimes lead to tremendous breakthroughs after the appropriate experimental technologies have been developed.
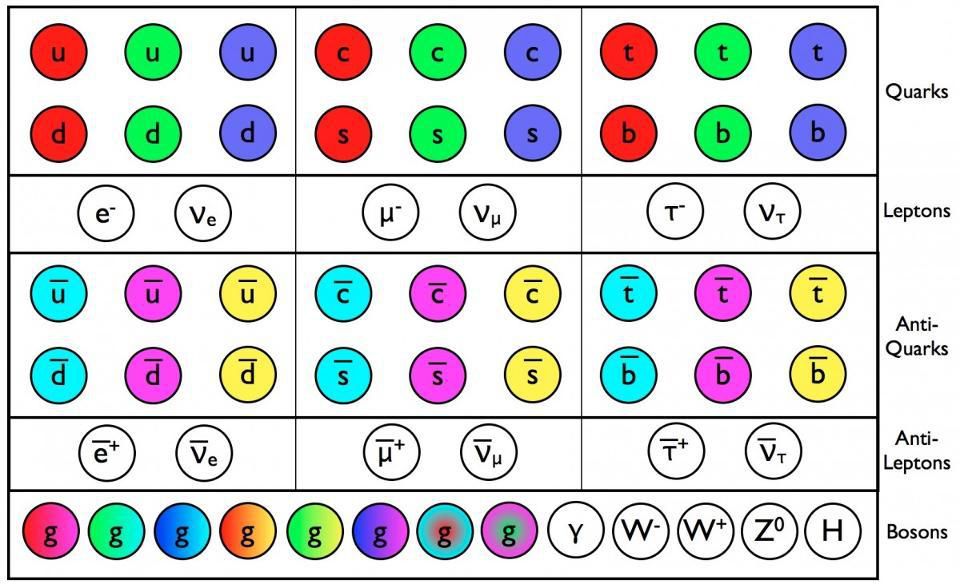
Quarks, antiquarks and gluons in the Standard Model have a color charge - in addition to other properties like mass and electric charge. All these particles, as far as we know, are punctate, and are distributed over three generations.
4) All particles with which we interact have unstable high-energy relatives. It is often said that scientific advances are usually met not by the exclamation of “eureka”, but by the remark “hmm, that’s strange ...” - but the first option was encountered in fundamental physics. If you charge an electroscope - in which two conductive metal sheets are connected to another conductor - both sheets will receive the same charge and will repel. If you put it in a vacuum, the sheets should not lose their charges, but they lose them over time. The best explanation for this was that high-energy particles, cosmic rays, fly from the outer space to the Earth, and the result of their collisions discharges an electroscope.

Astronomy of cosmic rays originated in 1912, when Victor Hess traveled in a balloon to the upper atmosphere and discovered particles falling onto the Earth from space.
In 1912, Victor Hess, using a balloon, conducted an experiment to search for these high-energy cosmic particles, and immediately discovered them in abundance, becoming the father of cosmic rays. By constructing a chamber with a magnetic field, one can measure the velocity and charge-to-mass ratio based on the curvature of the particle path. Protons, electrons, and even the first particles of antimatter were discovered that way, but the biggest surprise occurred in 1933, when Paul Kunz, working with cosmic rays, discovered the trail of a particle very similar to an electron, only hundreds of times heavier!

The first of the detected muons, along with other particles of cosmic rays, was the owner of the same charge as that of an electron, only with a mass hundreds of times more - this was evident from its speed and radius of curvature of the path
The existence of a muon with a lifetime of only 2.2 µs was later confirmed by experience when it was discovered by Karl Anderson and his student Seth Nedermayer, who used the ground-based Wilson chamber . When physicist Isidor Rabi , who himself won the Nobel Prize for the discovery of nuclear magnetic resonance, learned about the existence of a muon, he uttered the now famous phrase: “Who ordered it?” Later it was found that both composite particles (protons and neutrons) and fundamental ( quarks, electrons, neutrinos) possess several generations of heavier relatives, and the muon became the first of the open particles of the “second generation”.

The farther you look into space, the farther you look in time. In time you can not look further than 13.8 billion years: this is our estimate of the age of the universe. Extrapolating the data back to the earliest times led to the idea of the Big Bang.
5) The universe began with the Big Bang, but this discovery was made quite by accident. In the 1940s, Georgy Antonovich Gamov and his colleagues put forward a radical idea: the Universe, currently expanding and cooling, in the past was not only hotter and denser, but was arbitrarily hot and dense. If you extrapolate back far enough, you will have a universe that is hot enough to ionize all the matter in it, and even atomic nuclei will decay further. The idea became known as the Big Bang, and two major predictions came out of it:
1. In the Universe with which we started, there should have been not just protons and electrons, but a whole mixture of light elements synthesized together at high energies.
2. When the Universe has cooled enough to form neutral atoms, high-energy radiation is released and always travels in a straight line until it hits something, experiencing a red shift and losing energy as the universe expands.
They predicted that the temperature of this "background radiation" would be several degrees higher than absolute zero.
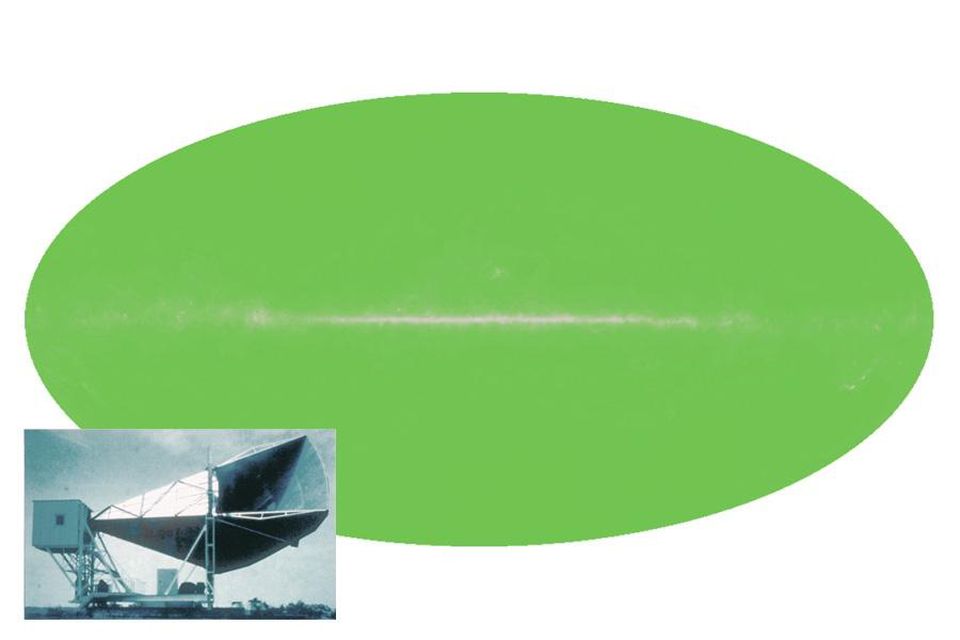
According to the original observations of Penzias and Wilson, there are several sources of radiation in the galactic plane (in the middle), but there was an almost perfectly uniform background above and below.
In 1964, Arno Penzias and Bob Wilson accidentally discovered the residual radiation of the Big Bang. Working with a radio antenna in Bell’s laboratories to study radar, they found that there was uniform noise coming from all places in the sky. It was not the Sun, not the Galaxy, not the atmosphere of the Earth - but they did not know what it was. They cleaned the surface of the antenna with rags, dispersed the pigeons, but the noise did not go anywhere. Only when the measurement results saw a physicist familiar with the detailed predictions of the Princeton group (Dick, Peebles, Wilkinson, etc.), and with the radiometer that was built just to detect a similar signal, did they realize the significance of what they found. For the first time, the origin of the universe became known.
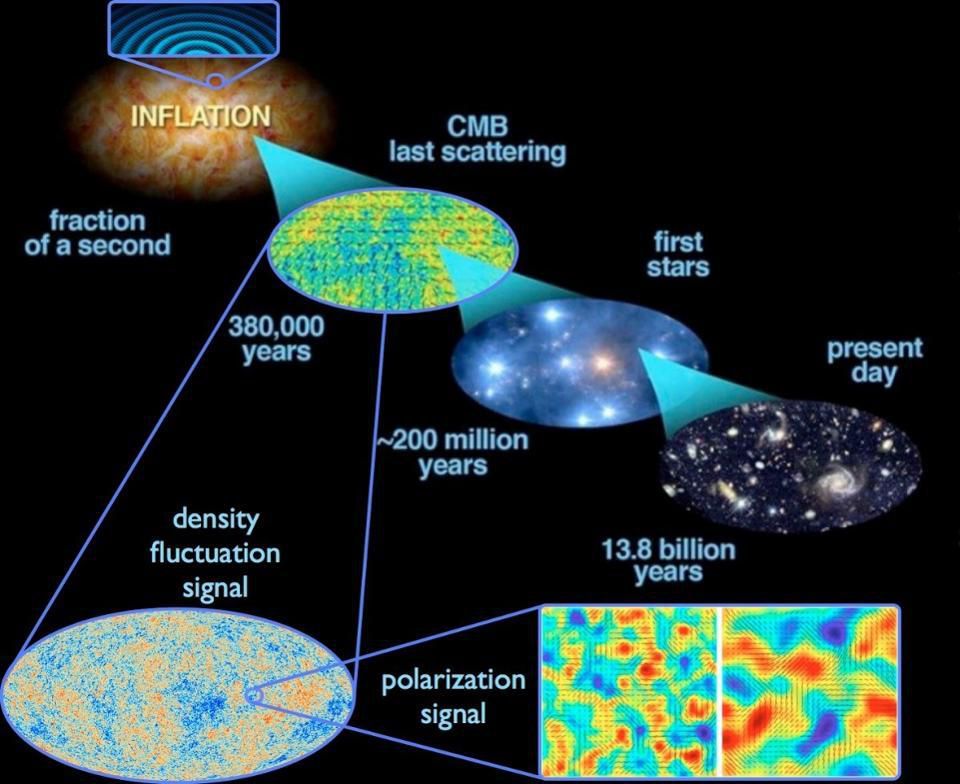
Quantum fluctuations inherent in space, stretched across the universe during cosmic inflation, and gave rise to stars, galaxies and other large-scale structures of the universe, known to us today. For 2017, this is the best idea of the origin of the structure and matter of the Universe.
Looking back, the scientific knowledge gathered to date, their predictive abilities, and how centuries of discoveries have transformed our lives can be tempted to look at science as a constant development of ideas. But in fact, the history of science is sloppy, full of surprises and burdened with disagreements. For those working on the frontier of modern knowledge, science is the risks, the study of new scenarios, attempts to go in an unknown direction. The story that remains in our memory is full of success, but the real story is full of dead ends, bad experiences and obvious mistakes. However, an open mind, a desire and the ability to test ideas, our ability to learn from the results and review our conclusions leads us from darkness to light. And in the end, everyone benefits from this.
Source: https://habr.com/ru/post/410925/
All Articles