How atoms work
What keeps an electron in an atom in the orbit of an atomic nucleus?
At first glance, especially if you look at the cartoon version of the atom, which I described earlier with all its flaws, the electrons moving in orbit around the nucleus look just like planets moving in orbit around the Sun. And the principle of these processes seems to be the same. But there is a catch.
Pic 1
')
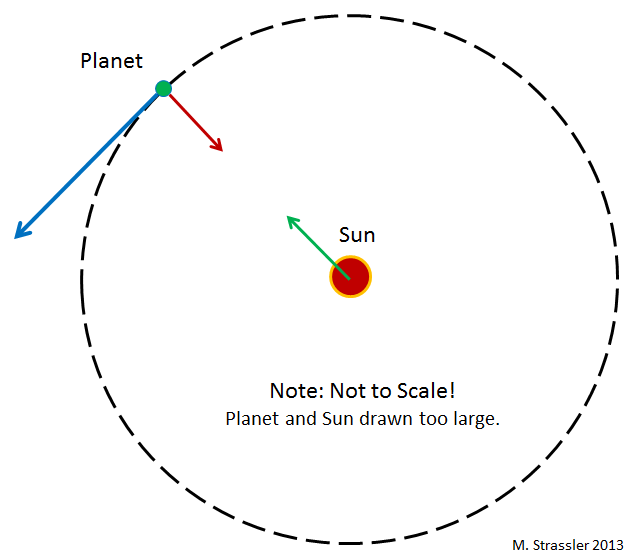
What keeps the planets in orbit around the sun? In Newtonian gravity (Einstein’s more complicated, but here we don’t need it) any pair of objects is attracted to each other through gravitational interaction, proportional to the product of their masses. In particular, the gravity of the Sun attracts planets to it (with a force inversely proportional to the square of the distance between them. That is, if the distance decreases by half, the force increases fourfold). Planets also attract the Sun, but it is so heavy that it almost does not affect its movement.
Inertia, the tendency of objects to move along straight lines in the absence of other forces acting on them, works against gravitational attraction, and as a result the planets move around the Sun. This is seen in Figure 1, where a circular orbit is depicted. Usually these orbits are elliptical - although in the case of planets they are almost round, since the Solar system was formed in this way. For various small stones (asteroids) and blocks of ice (comets) moving in orbits around the Sun, this is no longer the case.
Similarly, all pairs of electrically charged objects attract or repel each other, with a force that is also inversely proportional to the square of the distance between them. But, unlike gravity, which always attracts objects together, electric forces can both attract and repel. Objects with the same positive or negative charge are repelled. A negatively charged object attracts a positively charged object, and vice versa. Hence the romantic phrase “opposites attract”.
Therefore, a positively charged atomic nucleus in the center of the atom attracts lightweight electrons moving on the outskirts of the atom to itself, just like the sun attracts the planets. The electrons also attract the nucleus, but the mass of the nuclei is so much more that their attraction almost does not affect the nucleus. Electrons also repel each other, which is one of the reasons why they don’t like to spend time close to each other. It could be considered that electrons in an atom move in orbits around the nucleus in much the same way as planets move around the Sun. And at first glance, this is exactly what they do, especially in the cartoon atom.
But here is the trick: in fact, this is a double trick, and each of the two tricks has the opposite effect to the other, with the result that they are mutually destroyed!
Double trick: how atoms differ from planetary systems
Pic 2
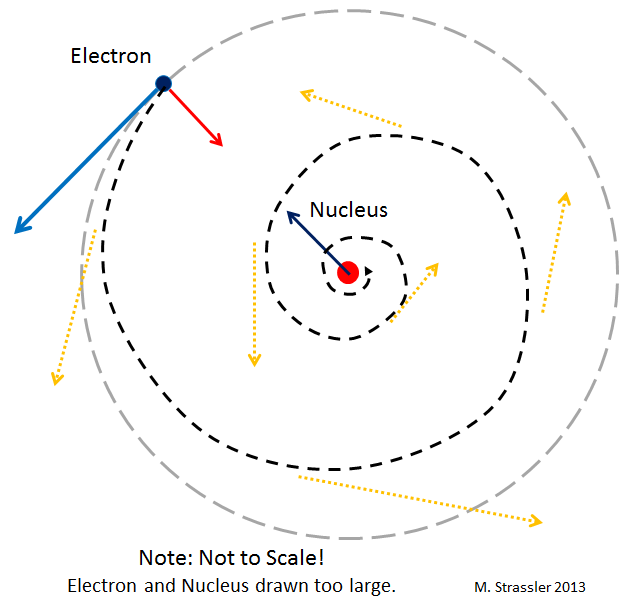
The first trick: unlike planets, electrons moving in orbits around the nucleus must emit light (more precisely, electromagnetic waves, one example of which is light). And this radiation should cause electrons to slow down and spiral down onto the nucleus. In principle, in Einstein's theory there is a similar effect - the planets can emit gravitational waves. But it is extremely small. Unlike the case of electrons. It turns out that the electrons in the atom must very quickly, in a small fraction of a second, spiral down to the nucleus!
And they would have done so if not for quantum mechanics. The potential catastrophe is shown in fig. 2
The second catch: but our world works according to the principles of quantum mechanics! And she has her own amazing and counterintuitive principle of uncertainty. This principle, which describes the fact that electrons are waves just like particles, deserves its own article. But here's what we need to know about it for today's article. The general consequence of this principle is that it is impossible to know all the characteristics of an object at the same time. There are sets of characteristics for which the measurement of one of them makes the others uncertain. One of the cases is the location and speed of particles such as electrons. If you know exactly where the electron is, you do not know where it is going, and vice versa. You can reach a compromise and with some accuracy know where it is, and with some accuracy know where it goes. In the atom, so it turns out.
Suppose an electron falls in a spiral on the nucleus, as in Fig. 2. In the process of his fall, we will know more and more precisely his location. Then the uncertainty principle tells us that its speed will become more and more uncertain. But if the electron stops at the nucleus, its speed will not be undefined! Therefore, he can not stop. If he suddenly tries to fall down in a spiral, he will have to move faster and faster randomly. And this increase in speed will lead the electron away from the nucleus!
So the tendency of falling in a spiral will be neutralized by the tendency towards faster movement according to the principle of uncertainty. The balance is when the electron is at a preferred distance from the nucleus, and this distance determines the size of the atoms!
Pic 3
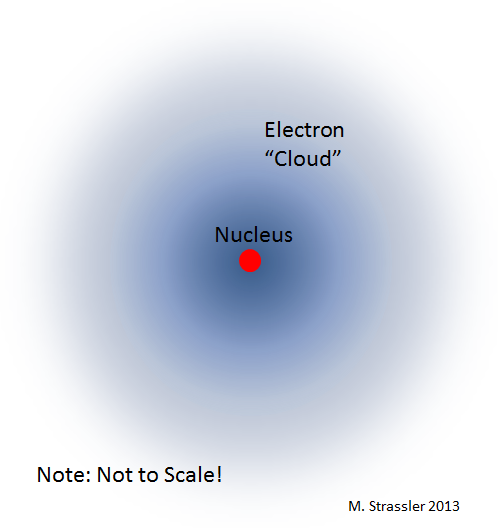
If the electron is initially located far from the nucleus, it will move to it in a spiral, as shown in Fig. 2, and emit electromagnetic waves. But as a result, its distance from the nucleus will be sufficiently small so that the uncertainty principle prohibits further convergence. At this stage, when a balance between radiation and uncertainty is found, the electron organizes a stable “orbit” around the nucleus (more precisely, the orbital - this term is chosen to emphasize that, unlike planets, the electron does not have such orbits because of quantum mechanics have planets). The radius of the orbital determines the radius of the atom (Fig. 3).
Another feature - the belonging of electrons to fermions - causes electrons not to descend to one radius, and line up along orbitals of different radii.
How large are atoms? Approximation based on uncertainty principle
In fact, we can roughly estimate the size of an atom using only calculations for electromagnetic interactions, the mass of an electron and the principle of uncertainty. For simplicity, we will do the calculations for a hydrogen atom, where the nucleus consists of one proton, around which one electron moves.
The uncertainty principle states:
where ℏ is Planck's constant h divided by 2 π. Note that he says that (Δ v) (Δ x) cannot be too small, which means that both determinations cannot be too small, although one of them may be very small if the other is very large.
When an atom is established in the preferred ground state, we can expect the sign ≥ to turn into ~, where A ~ B means that “A and B are not exactly equal, but not very different”. This is a very useful symbol for ratings!
For a hydrogen atom in the ground state, in which the uncertainty of the position Δx will be approximately equal to the radius of the atom R, and the uncertainty of the speed Δv will be approximately equal to the typical speed V of the electron moving around the atom, we get:
How to learn R and V? There is a relationship between them and the force that holds the atom together. In non-quantum physics, an object of mass m located on a circular orbit of radius r and moving at a speed v around a central object attracting it with a force F will satisfy the equation
This is not applicable to an electron in an atom directly, but it works approximately. The force acting in an atom is the electric force with which a proton with a charge +1 attracts an electron with a charge of -1, and as a result the equation takes the form
where k is the Coulomb constant, e is the unit of charge, c is the speed of light, ℏ is Planck's constant h divided by 2 π, and α is the fine structure constant we have defined fracke2ℏc sim1/137.04 . We combine the two previous equations for F, and the estimated ratio is as follows:
Now apply this to the atom, where v → V, r → R, and m → m e . Also multiply the upper equation by meR3 . This gives:
In the last step, we used our uncertainty relation for the atom, meVR simℏ . Now you can calculate the radius of the atom R:
And it turns out to be almost accurate! Such simple estimates will not give you accurate answers, but they will provide a very good approximation!
At first glance, especially if you look at the cartoon version of the atom, which I described earlier with all its flaws, the electrons moving in orbit around the nucleus look just like planets moving in orbit around the Sun. And the principle of these processes seems to be the same. But there is a catch.

Pic 1
')
What keeps the planets in orbit around the sun? In Newtonian gravity (Einstein’s more complicated, but here we don’t need it) any pair of objects is attracted to each other through gravitational interaction, proportional to the product of their masses. In particular, the gravity of the Sun attracts planets to it (with a force inversely proportional to the square of the distance between them. That is, if the distance decreases by half, the force increases fourfold). Planets also attract the Sun, but it is so heavy that it almost does not affect its movement.
Inertia, the tendency of objects to move along straight lines in the absence of other forces acting on them, works against gravitational attraction, and as a result the planets move around the Sun. This is seen in Figure 1, where a circular orbit is depicted. Usually these orbits are elliptical - although in the case of planets they are almost round, since the Solar system was formed in this way. For various small stones (asteroids) and blocks of ice (comets) moving in orbits around the Sun, this is no longer the case.
Similarly, all pairs of electrically charged objects attract or repel each other, with a force that is also inversely proportional to the square of the distance between them. But, unlike gravity, which always attracts objects together, electric forces can both attract and repel. Objects with the same positive or negative charge are repelled. A negatively charged object attracts a positively charged object, and vice versa. Hence the romantic phrase “opposites attract”.
Therefore, a positively charged atomic nucleus in the center of the atom attracts lightweight electrons moving on the outskirts of the atom to itself, just like the sun attracts the planets. The electrons also attract the nucleus, but the mass of the nuclei is so much more that their attraction almost does not affect the nucleus. Electrons also repel each other, which is one of the reasons why they don’t like to spend time close to each other. It could be considered that electrons in an atom move in orbits around the nucleus in much the same way as planets move around the Sun. And at first glance, this is exactly what they do, especially in the cartoon atom.
But here is the trick: in fact, this is a double trick, and each of the two tricks has the opposite effect to the other, with the result that they are mutually destroyed!
Double trick: how atoms differ from planetary systems

Pic 2
The first trick: unlike planets, electrons moving in orbits around the nucleus must emit light (more precisely, electromagnetic waves, one example of which is light). And this radiation should cause electrons to slow down and spiral down onto the nucleus. In principle, in Einstein's theory there is a similar effect - the planets can emit gravitational waves. But it is extremely small. Unlike the case of electrons. It turns out that the electrons in the atom must very quickly, in a small fraction of a second, spiral down to the nucleus!
And they would have done so if not for quantum mechanics. The potential catastrophe is shown in fig. 2
The second catch: but our world works according to the principles of quantum mechanics! And she has her own amazing and counterintuitive principle of uncertainty. This principle, which describes the fact that electrons are waves just like particles, deserves its own article. But here's what we need to know about it for today's article. The general consequence of this principle is that it is impossible to know all the characteristics of an object at the same time. There are sets of characteristics for which the measurement of one of them makes the others uncertain. One of the cases is the location and speed of particles such as electrons. If you know exactly where the electron is, you do not know where it is going, and vice versa. You can reach a compromise and with some accuracy know where it is, and with some accuracy know where it goes. In the atom, so it turns out.
Suppose an electron falls in a spiral on the nucleus, as in Fig. 2. In the process of his fall, we will know more and more precisely his location. Then the uncertainty principle tells us that its speed will become more and more uncertain. But if the electron stops at the nucleus, its speed will not be undefined! Therefore, he can not stop. If he suddenly tries to fall down in a spiral, he will have to move faster and faster randomly. And this increase in speed will lead the electron away from the nucleus!
So the tendency of falling in a spiral will be neutralized by the tendency towards faster movement according to the principle of uncertainty. The balance is when the electron is at a preferred distance from the nucleus, and this distance determines the size of the atoms!

Pic 3
If the electron is initially located far from the nucleus, it will move to it in a spiral, as shown in Fig. 2, and emit electromagnetic waves. But as a result, its distance from the nucleus will be sufficiently small so that the uncertainty principle prohibits further convergence. At this stage, when a balance between radiation and uncertainty is found, the electron organizes a stable “orbit” around the nucleus (more precisely, the orbital - this term is chosen to emphasize that, unlike planets, the electron does not have such orbits because of quantum mechanics have planets). The radius of the orbital determines the radius of the atom (Fig. 3).
Another feature - the belonging of electrons to fermions - causes electrons not to descend to one radius, and line up along orbitals of different radii.
How large are atoms? Approximation based on uncertainty principle
In fact, we can roughly estimate the size of an atom using only calculations for electromagnetic interactions, the mass of an electron and the principle of uncertainty. For simplicity, we will do the calculations for a hydrogen atom, where the nucleus consists of one proton, around which one electron moves.
- We denote the electron mass me
- The electron position uncertainty is denoted by Δx
- The electron velocity uncertainty is denoted by Δv
The uncertainty principle states:
$$ display $$ m_e (Δ v) (Δ x) ≥ ℏ $$ display $$
where ℏ is Planck's constant h divided by 2 π. Note that he says that (Δ v) (Δ x) cannot be too small, which means that both determinations cannot be too small, although one of them may be very small if the other is very large.
When an atom is established in the preferred ground state, we can expect the sign ≥ to turn into ~, where A ~ B means that “A and B are not exactly equal, but not very different”. This is a very useful symbol for ratings!
For a hydrogen atom in the ground state, in which the uncertainty of the position Δx will be approximately equal to the radius of the atom R, and the uncertainty of the speed Δv will be approximately equal to the typical speed V of the electron moving around the atom, we get:
meVR simℏ
How to learn R and V? There is a relationship between them and the force that holds the atom together. In non-quantum physics, an object of mass m located on a circular orbit of radius r and moving at a speed v around a central object attracting it with a force F will satisfy the equation
F= fracmv2r
This is not applicable to an electron in an atom directly, but it works approximately. The force acting in an atom is the electric force with which a proton with a charge +1 attracts an electron with a charge of -1, and as a result the equation takes the form
F= fracke2r2= fracαℏcr2
where k is the Coulomb constant, e is the unit of charge, c is the speed of light, ℏ is Planck's constant h divided by 2 π, and α is the fine structure constant we have defined fracke2ℏc sim1/137.04 . We combine the two previous equations for F, and the estimated ratio is as follows:
fracαℏcr2 sim fracmv2r
Now apply this to the atom, where v → V, r → R, and m → m e . Also multiply the upper equation by meR3 . This gives:
αℏcmeR simme2V2R2=(meVR)2 simℏ2
In the last step, we used our uncertainty relation for the atom, meVR simℏ . Now you can calculate the radius of the atom R:
R sim fracℏαcme sim frac137(10−34kgm2/s)(3•108m/s•9•10−31kg)sim0.5•10−10m
And it turns out to be almost accurate! Such simple estimates will not give you accurate answers, but they will provide a very good approximation!
Source: https://habr.com/ru/post/403947/
All Articles