A cheaper method of artificial photosynthesis has been invented.

For many years, scientists have been struggling with the problem of economically viable artificial photosynthesis. The goal is to effectively use the free energy of sunlight for chemical reactions. So far, it has been possible to use high-energy ultraviolet rays for this purpose, but they constitute only 4% of the spectrum of sunlight. For the other parts of the spectrum, only a few effective materials have been found so far, but they require expensive additives: platinum ($ 31 per gram), rhenium ($ 1000 per gram) or iridium ($ 35 per gram).
Chemistry professor Fernando Uribe-Romo (Fernando Uribe-Romo) from the University of Central Florida with his students has developed a new synthetic material that converts CO 2 into fuel under the influence of photons of light. Such material solves two problems at once: it reduces the amount of greenhouse gas and gives “environmentally friendly” fuel. And the most important thing is that precious metals are not needed for its manufacture! It uses titanium, which is sold in kilograms - it is almost a thousand times cheaper than platinum or iridium.
Synthetic material is a metal-organic framework structure (metal – organic framework, MOF). By the way, similar MOFs from Zr 6 O 4 (OH) 4 (fumarate) 6 ] are used to condense water from air , also with the help of sunlight alone. Imagine, even in the driest desert you put an empty bottle on the street - and it fills itself with water.
')
The illustration below shows the crystal structure of the metal-organic structure crystal MOF MIL-125 for artificial photosynthesis.
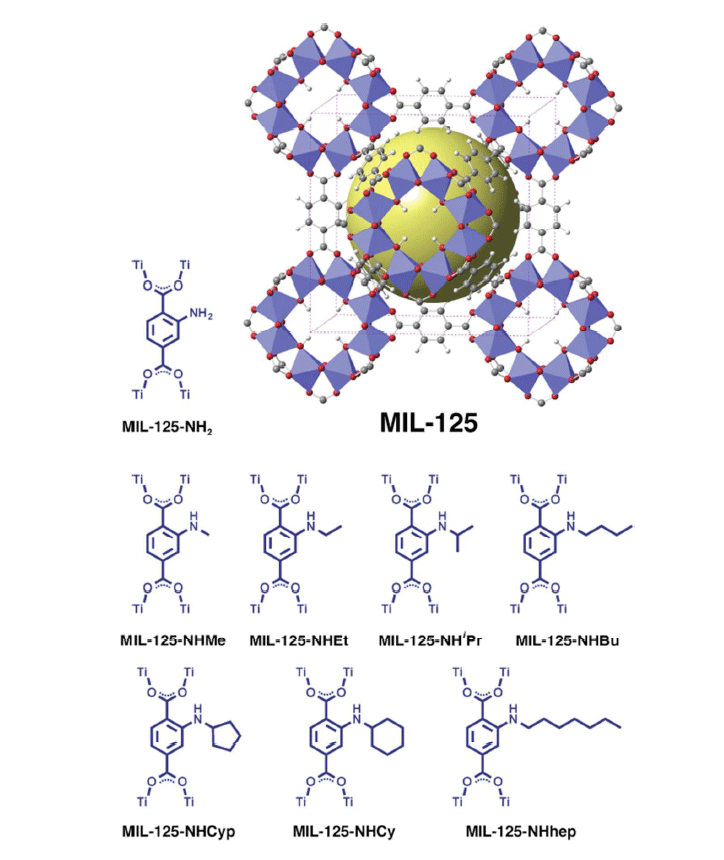
In this case, metal-organic structures also use photons of light for energy, but the output produces harmless organic substances - much like plants produce food and building material for themselves in the process of natural photosynthesis. The collection of solar energy is carried out by molecules called N-alkyl-2-aminoterephthalates. The authors of the research claim that the molecules can be modified to react to specific parts of the spectrum. In this case, the reaction to the blue color was used. When tested, the material was irradiated with blue LEDs.

Photoreactor. Photo: University of Central Florida
Carbon dioxide was pumped into a kind of “photoreactor” with a powerful LED backlight. As a result of photosynthesis in MOF, carbon dioxide was converted to formates and formamides. Formates are formic acid salts, and formamides are formic acid amides. This is a kind of solar fuel.
Fernando Uribe-Romo is going to continue his research: he wants to study the effectiveness of artificial photosynthesis at different wavelengths, as well as with a higher concentration of carbon dioxide, where the efficiency of photosynthesis should increase. The professor says that if the process works efficiently, this material will help in reducing the level of greenhouse gases in the atmosphere and will be suitable for a clean method of producing fuel. “This work is a breakthrough,” says the author. “Making materials to absorb a certain spectrum of light is very difficult from a scientific point of view, but from a social point of view, we are helping to develop technology that can reduce the level of greenhouse gases.”

Professor Fernando Uribe-Romo. Photo: University of Central Florida
Maybe in the future they will start building power plants of a new type - whole factories, which will start to suck carbon dioxide from the atmosphere in large quantities and produce fuel. For lack of CO 2, you can not worry - it is extremely easy to fill in, so green plants on Earth always have enough material for life.
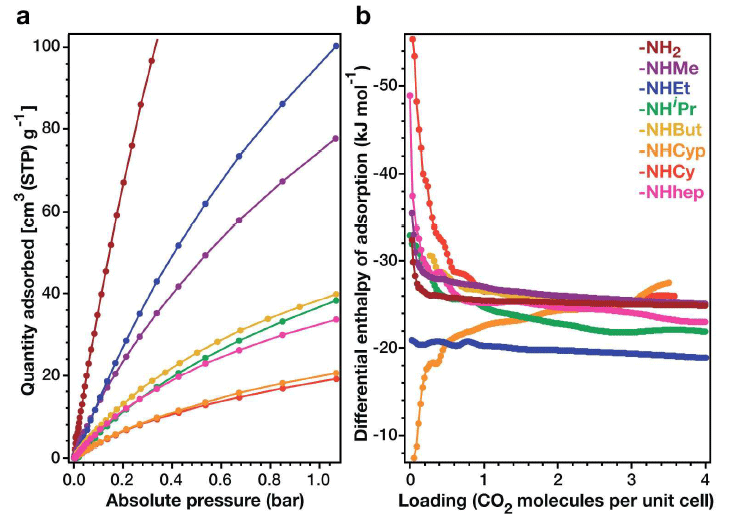
On the left - CO 2 absorption isotherms (273 K), on the right - the enthalpy differential of absorption
The professor even imagines that in the future people will begin to cover the roofs of houses with such metal-organic material, thereby producing energy for the household and cleaning the air in the yard.
The scientific work was published on April 7, 2017 in the Journal of Materials Chemistry A (doi: 10.1039 / C7TA00437K, pdf ).
Source: https://habr.com/ru/post/403517/
All Articles