How were the first atoms discovered in the universe
We do not know how a star appears, but we want to find out how 10 billion stars appear.
- Carlos Frank
Looking into the remote parts of the universe, we look into its past. The farther the object, the longer its light went to our eyes. And every time when we manage to look further than it was before, we look into a deeper past - closer and closer to the Big Bang.
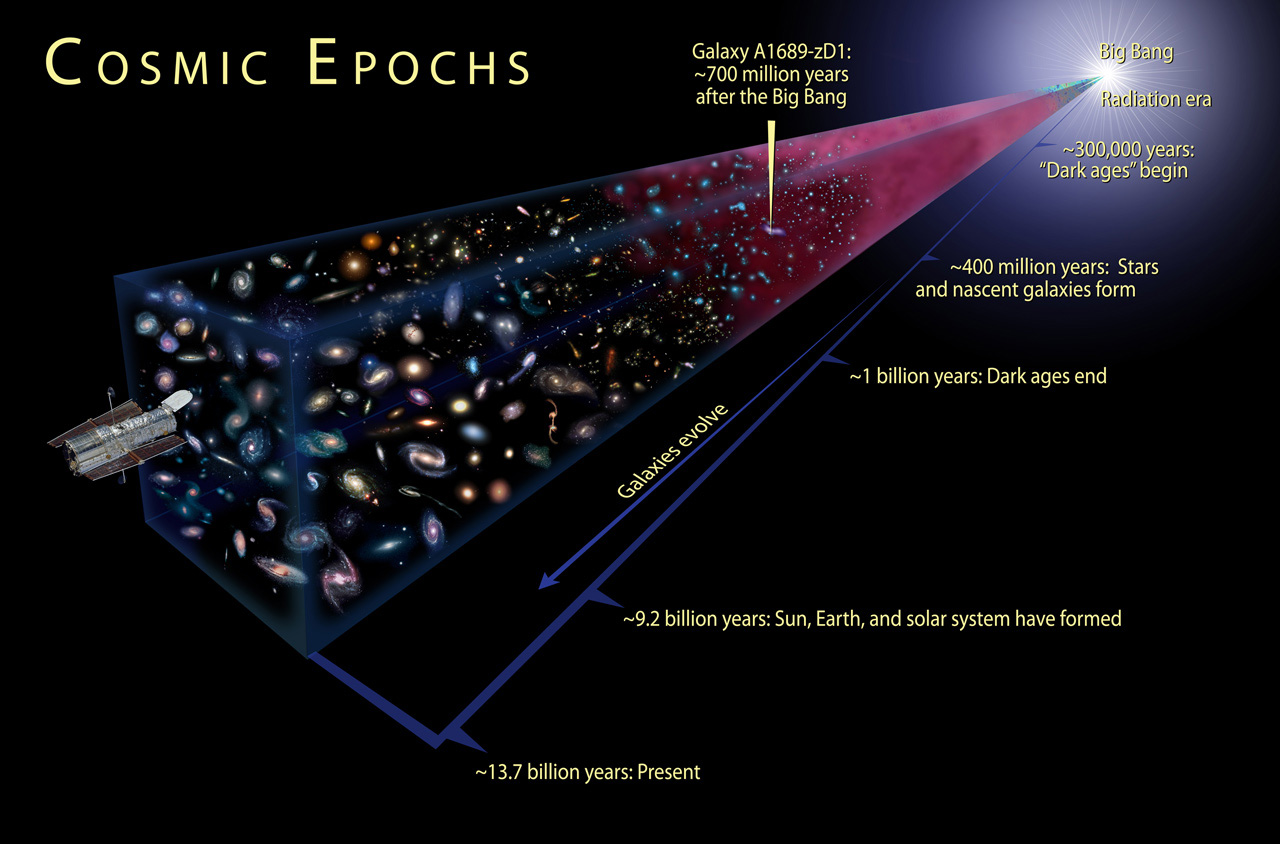
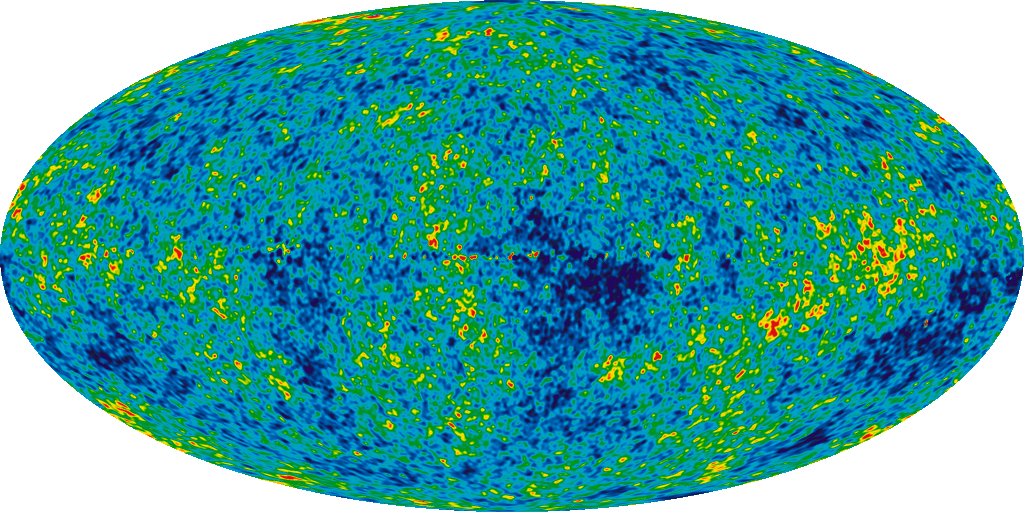
The earliest we have seen is, of course, relic radiation, the residual luminescence from the Big Bang. When we observe this background radiation emitted while the Universe has finally cooled to temperatures that allow atoms to form, we get a picture of the Universe at the age of 380,000 years!
')
But there is a theoretical prediction concerning the Big Bang, originating from even earlier times. This is perhaps the earliest of all predictions that you can check! The big bang not only shows when the atoms should have been formed for the first time, but also what kind of atoms they should have been.
What is this way? Fast forward to the earliest stages, about which we can argue, and at which we are still 100% sure of the correctness of physics.
Recall that the Universe is expanding and cooling, which means that it was hotter and denser in the past! Of course, when the universe was less than 380,000 years old, it was too hot for neutral atoms, but what if we go even further?
At some point it was too hot and dense even for nuclei, and even earlier - too energetic for the existence of protons and neutrons! When the age of the Universe did not exceed a small fraction of a second, we only had a sea of quarks, gluons, leptons, anti-blinds and super-hot radiation, and it all swam in the primary soup of the early Universe!
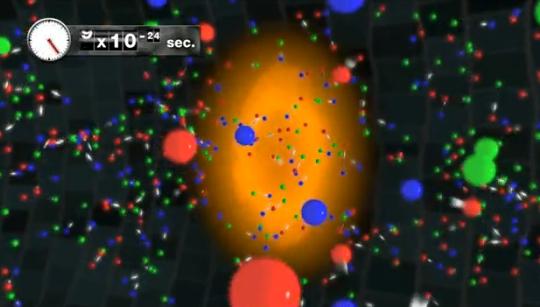
In this state, everything collides extremely quickly and is in thermal equilibrium. The creation and annihilation of particle / antiparticle pairs occurs very quickly. However, almost all particles are unstable. With the expansion and cooling of the universe, heavy leptons and quarks decay, excess matter and antimatter occur and annihilate, and the remaining quarks (upper and lower in approximately equal quantities) cool enough to condense into separate protons and neutrons. By the time the age of the Universe reaches 10 microseconds, there are approximately equal numbers of protons and neutrons.

However, the Universe is also filled with electrons and antielectrons, better known as positrons. Every time a proton collides with a sufficiently energetic electron, a neutron (and neutrino) is born, and every time a neutron collides with a sufficiently energetic positron, a proton (and antineutrino) is born. Initially, these reactions take place at about the same speed, and we get the Universe with normal matter, 50% composed of protons and 50% neutrons.
But due to the fact that protons are lighter than neutrons, it becomes more profitable to energetically increase the number of protons and reduce the number of neutrons . By that time, when the age of the Universe is 3 seconds and all transformations have practically stopped, 85% of protons and 15% of neutrons are already in the Universe. And at that time it is still hot enough and dense enough for protons and neutrons to try to launch nuclear fusion of deuterium, the first heavy isotope of hydrogen!

But in the universe there are more than a billion photons per proton or neutron, and the temperature is still too high for the deuterium to be immediately destroyed. So we wait, wait, and wait until the Universe cools down to create a deuterium and not crash it immediately. In the meantime, the trouble is that the neutron is unstable, and some neutrons break up into protons, electrons and antineutrinos.
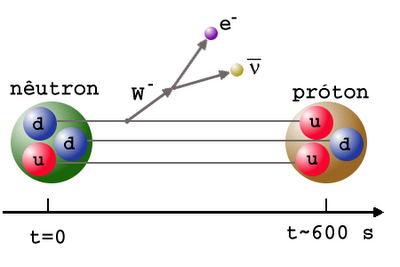
Finally, between the 3rd and 4th minute of the Universe, the photons are cooled sufficiently so as not to smash deuterium faster than protons and neutrons can produce. The universe passes through a bottleneck associated with deuterium. At this moment, thanks to decays, there are 88% of protons and 12% of neutrons in the Universe.
When the Universe begins to produce deuterium, it immediately adds protons and / or neutrons to it, climbing the ladder of elements to tritium or helium-3, and then to extremely stable helium-4!

Almost all neutrons were in atoms of helium-4, constituting 24% of all atoms by weight after this nucleosynthesis. The nuclei of hydrogen - just individual protons - accounted for the remaining 76%. There was also a small proportion (from 0.001% to 0.01%) of helium-3, tritium (decaying to helium-3) and deuterium, and an even smaller proportion of different forms of lithium and beryllium, which appeared as a result of nucleosynthesis with a helium-4 core.
But due to a combination of factors - the lack of stable nuclei with a mass of 5 or 8, a relatively low temperature and density of the Universe by this time, and a strong electrical repulsion of heavy isotopes - nothing heavier has formed.
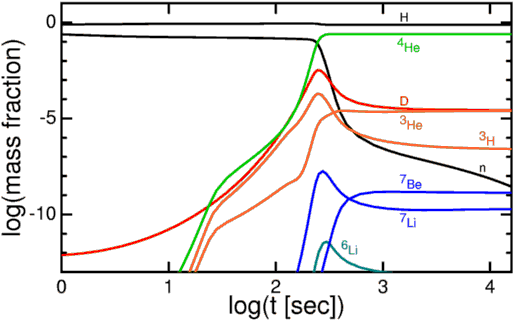
And such elements were predicted by the Big Bang theory. With our knowledge of relic radiation, we can determine - with incredible accuracy - how much helium-4, helium-3, deuterium and lithium-7 should be in our day. This prediction — the initial abundance of light elements — is one of the greatest predictions that emerged from the Big Bang model.
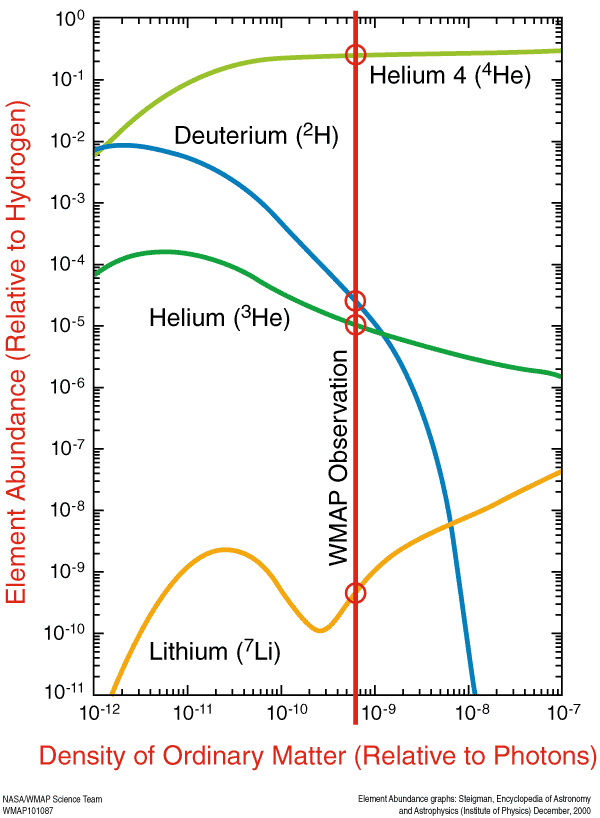
After that, the Universe simply expands and cools, and unstable isotopes (such as tritium) decompose into stable ones until these atomic nuclei — created in the nuclear furnace of the Big Bang — catch electrons and become neutral atoms.
Of course, seeing these atoms and measuring their abundance is a particularly difficult task. Why? Let's look at what you can see if you look into the early Universe.

We want to see the very first atoms: those that existed in dark times of space. But it is extremely difficult.
We determine the presence of elements in the universe from their atomic transitions. They either show emission lines, if the atoms are hot enough, and their excited electrons move to a lower energy state, or absorption lines, if the atoms are in a cold state with low energy, but there is a hot source behind them, whose photons are absorbed at the right level of energy. by atoms.
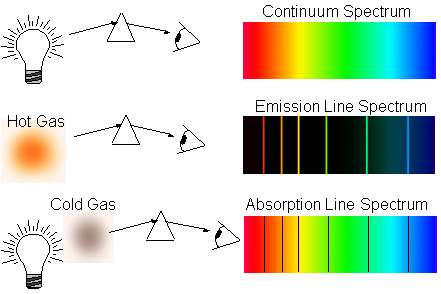
The problem, of course, is that these atoms of the "dark ages" are too cold in themselves to produce emission lines, and the radiation behind them is too weak to cause absorption lines! Therefore, we have to wait until gravity does its job and draws enough of them to one place so that we can use something energetic enough to cause absorption lines in them!
After a fairly strong gravitational collapse, the Universe in places becomes dense enough to form stars for the first time! Regions that become dense faster than others form the stars first - 50-150 Ma after the Big Bang - and other regions remain neutral, star-free and untouched.
The first problem is that when these first stars are created, the light from them is blocked by neutral atoms, just like starlight is blocked by a dense cloud of interstellar gas.

Therefore, we need, if we want to see the light of these stars (or any source of light), to get rid of these neutral atoms. For this it is necessary to form enough stars in the Universe in order to re-ionize most of the (99% +) neutral atoms. Fortunately, the Universe has been doing this on its own, and in less than a billion years.
Another problem is that when a gravitational collapse occurs, and the first stars appear, they not only litter the Universe with heavy elements created by them, but also destroy these meager light elements - deuterium, lithium, helium-3 - which we want to measure!
So you might think that trick-22 works here. How can we measure these first, untouched atoms, if we can measure them only after a billion years, when everything that happens pollutes the atoms of the universe?
But there is hope.

In the Universe, there are - although they are difficult to find - isolated ultra-small mass galaxies, such as the Pump dwarf galaxy (from the constellation Pump ), shown above.
Theoretically, extremely isolated pieces of matter, whose mass is approximately 0.0001% of the mass of our Milky Way galaxy, could survive without forming any stars at all, and not be contaminated by the post-star mass that was next to them for more than a billion years. But to find such a piece, we had to be very lucky.
Well, we were lucky exactly as we hoped.

The brightest and lightest objects visible on the distant outskirts of the universe are quasars , most of which are visible at the very last stage of reionization — when matter becomes transparent to light — in the universe. After 58 years of spectroscopic study of quasars , the fumagali, Omear and Prochask team found a lucky chance to detect two clouds of untouched, unpolluted gas, preserved from the Big Bang, in quasar spectra!

On the upper part of the image taken from the work of Fumagali and others , the spectrum of the quasar is depicted. A dip on a zigzag chart is a sign of a takeover line! In this case, the absorption lines show the characteristics of a neutral hydrogen gas cloud with a red shift slightly more than 3, that is, in time somewhere 2 billion years after the Big Bang (and approximately 1 billion years after the first light left this quasar ). However, signs of vital activity of previous stars are usually present - such “polluting” elements as carbon, oxygen, silicon, etc. - not just a little, but very little, less than 0.01% of the amount contained in our Sun. This is if we consider that the next cloud of “purity” that we found in the Universe already contains more than 0.1% of the number of heavy elements in the Sun.
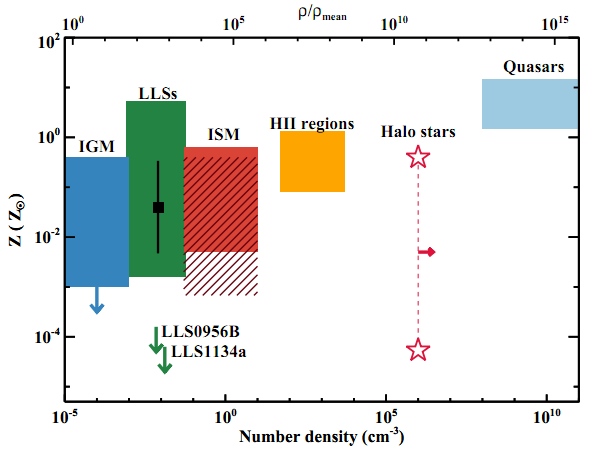
So, this is not only the least polluted and most intact set of atoms we found, it is also the best of all tests that the abundance of light elements - judging by the strength of their spectral absorption lines - coincides with the predictions of the Big Bang theory!
What are the results? Take a look at the most untouched, leftmost point on the graph; This is the most reliable data we have ever received on this topic!
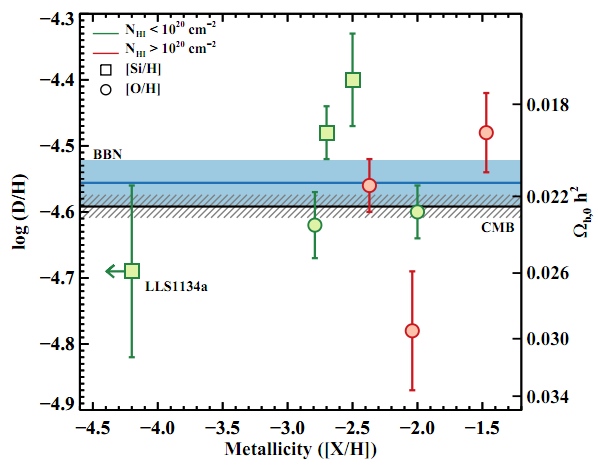
The paper says:
On the line of sight of quasars, the measured log (D / H) = −4.55 ± 0.03 is recalculated into Ωb, 0h2 (BBN) = 0.0213 ± 0.0010, which completely coincides with the amount following from the power spectrum of the background radiation, Ωb, 0h2 (CMB) = 0.02249 ± 0.00057. This beautiful coincidence between two independent experiments marks the triumph of the Big Bang theory.
And what is best - if we want to better measure the elements found in these clouds of gas, we just need to study them a little more time! Yes, we can get lucky and we can find even more of these intact gas clouds (the rule of thumb says that one case is an accident, and two is a possible pattern), but even if we don’t find them, we just need to look at these quasars, and we can even better clarify the number of elements in them!
That's how we found the very first atoms of the Universe, and how they proved the validity of another prediction of the Big Bang theory.
Source: https://habr.com/ru/post/402215/
All Articles