Genetic engineering of bacteria: how to bring the genes we need as part of a plasmid vector into a bacterium
In my previous publication , two questions were considered: the basics of molecular biology and how to create plasmid vectors based on the genes we need. Now you need to figure out how to put a plasmid vector into a bacterial cell, that is, to make a "transformation". At the same time, we will learn something about the structure of the bacterial membrane and how it can be overcome, as well as why some bacteria are called competent, how all this is connected with bacterial toxins and antibiotic resistance of bacteria and many other interesting facts.
So, we have a plasmid vector on hand and there is a bacterial strain suitable for this vector, it remains to add the vector to the bacterium.
The previous article mentioned the work of Frederick Griffith , which clearly demonstrates the fact that DNA can spontaneously penetrate bacterial cells. The ability of a bacterial cell to absorb the environmentally located DNA molecule is called “competence” (which gives rise to a lot of jokes about the fact that even single-celled in vitro is competent, and your laboratory colleague is not). If absorption occurs not under the influence of some artificially created conditions, then we are talking about “natural competence”. How does the similar DNA transport inside the bacteria occur?
To begin, consider briefly the structure of the shell of gram-positive and gram-negative bacteria.
The shell of a gram-negative bacterium consists of two lipid bilayers: the outer membrane and the inner membrane (the inner one is also called the plasmatic membrane). Between them there is a periplasmic space filled with important proteins and peptidoglycan.
')
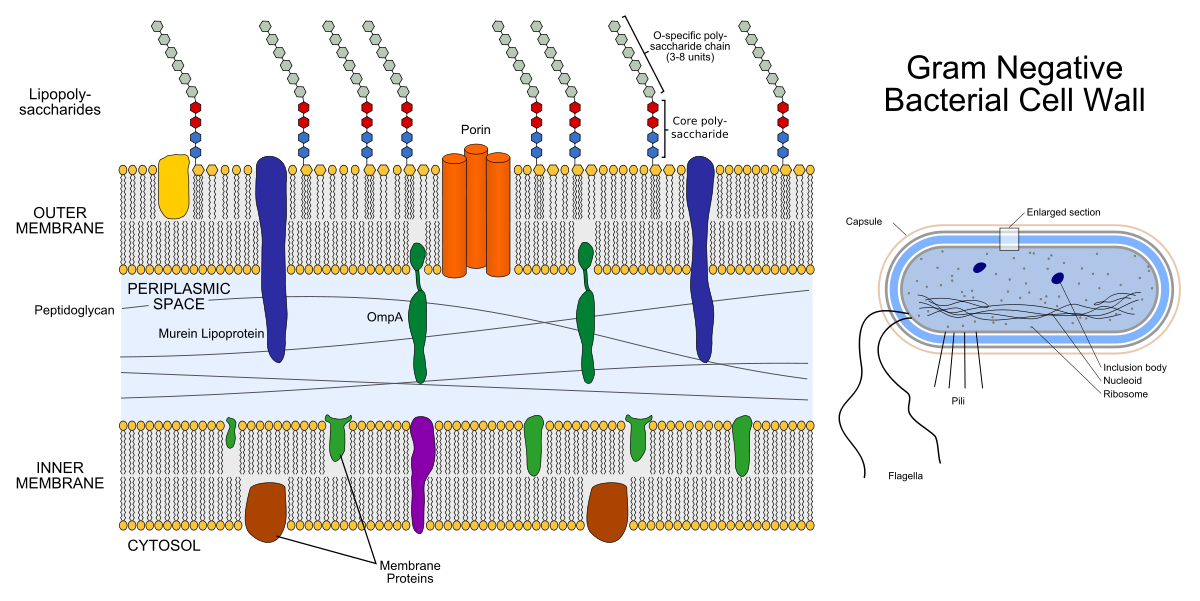
On the left - the structure of the shell of gram-negative bacteria. The colored things inside the membranes are membrane proteins, and the chains sticking outward are lipopolysaccharides. On the right - the structure of the most gram-negative bacteria.
A peptidoglycan is a heteropolymer consisting of covalently crosslinked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). It turns out the chain ....- NAG-NAM-NAG-NAM-NAG- .... “Hetero” in the word “heteropolymer” means that the polymer consists not of the same elements (as polyethylene), but of two different. The synthesizing peptidoglycan transpeptidase enzyme is a target for β-lactam antibiotics (penicillin antibiotics and cephalosporins): they suppress its activity, as a result of which the peptidoglycan is not sufficient for both daughter cells. The peptidoglycan layer is also a target for the lysozyme enzyme: it breaks the covalent bond between NAG and NAM. In medicine, lysozyme is used as an antiseptic, for example, it is an active component of some drugs for treating sore throat, and in the food industry it can be used as a preservative (food additive E1105 is lysozyme).

Peptidoglycan. The figure shows the part corresponding to one NAG (it is on the left) and one NAM (it is on the right), which are interconnected through an oxygen atom. (For those who do not like such liberties in chemistry: this "bond through an oxygen atom" is called β- (1,4) -glycosidic).
The shell of gram-positive bacteria consists only of the inner (plasma) membrane, a very thin periplasmic space and an outer, thicker peptide layer than the gram-negative bacteria.
Such obstacles that stand in the way of any compound into the bacterial cytoplasm are insurmountable for everything except water, as well as small non-polar and hydrophobic molecules (molecular oxygen and nitrogen, carbon dioxide and others). The penetration of these compounds through the envelope is called “passive transport” (“simple diffusion”); it always occurs along a concentration gradient. Non-polar molecules are able to freely pass through the membranes because the internal volume of the membranes is also non-polar (inside the membrane they consist of hydrophobic fatty acid residues), and non-polar compounds dissolve well in non-polar solvents (alcohol, acetone, fatty acids).
A comic picture in which the hydrophobicity (hydrophobia) of fatty acid residues inside a two-layer membrane is played up.
In turn, large polar molecules (amino acids, monosaccharides, nucleotides), charged particles (ions) and macromolecules (DNA, proteins) cannot penetrate through the cell membrane just like that, since they are well soluble only in polar solvents (for example, in water) . Then how do DNA molecules get inside the bacterial cell?
First of all, we have on our hands the obvious fact that the bacterium cannot live only on water, oxygen and carbon dioxide, it needs many other compounds for its vital activity. So there are ways to deliver these compounds into the cell. Such methods are “facilitated diffusion” and “active transport”.
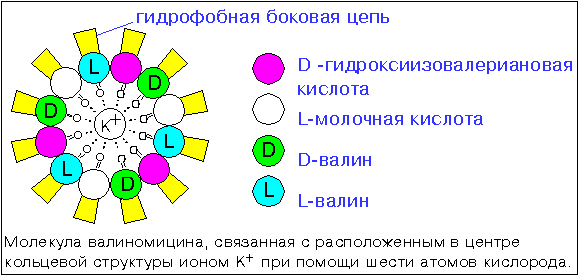
An example of facilitated diffusion (although this particular case is contrary to bacteria for bacteria) is the transport of potassium ions through the membrane of a bacterial cell in combination with valinomycin, an antibiotic that is synthesized by some fungi. Free valinomycin cannot penetrate inside the cell, since polar groups are exposed to it outside. Potassium ion is also "way closed."

The sculpture depicting the three-dimensional structure of the complex of valinomycin and potassium ion (ion depicted as a ball in the center). Installed in front of the entrance to the Institute of Bioorganic Chemistry of the Russian Academy of Sciences named after M. Shemyakin and Yu. A. Ovchinnikov (Moscow), which established the mechanism of antibacterial activity of valinomycin and the three-dimensional structure of its complex with potassium ion.
But after forming a complex of valinomycin with a potassium ion, it changes its three-dimensional structure in such a way that its entire outer surface becomes hydrophobic (non-polar), and all hydrophilic groups are hidden inside. At the same time, the potassium ion is hidden inside the molecule of valinomycin, therefore the complex “valinomycin + potassium ion” can penetrate through the membrane. The complex can break up to free valinomycin and an ion at any time, both inside the cell and outside, but in general, facilitated diffusion of ions leads to the equalization of their concentration both in the intracellular and extracellular space, and the cell controls the ion transport for a reason: high concentration destructive. This is the basis of the antibacterial effect of valinomycin.
Schematic representation of the fungal antibiotic valinomycin, which formed a complex with potassium ion.
Another example is uncontrolled pores, which are large transmembrane proteins that allow certain substances to pass unhindered through the membrane according to a gradient. An example of such a transmembrane pore is the α-toxin of Staphylococcus aureus Staphylococcus aureus (another name for the toxin: α-hemolysin. It consists of “hemo” and “lysine” parts. “Hemo” means red blood cells are the target of the toxin; red blood cells; lysine - from the word "lysis", that is, "cell death")
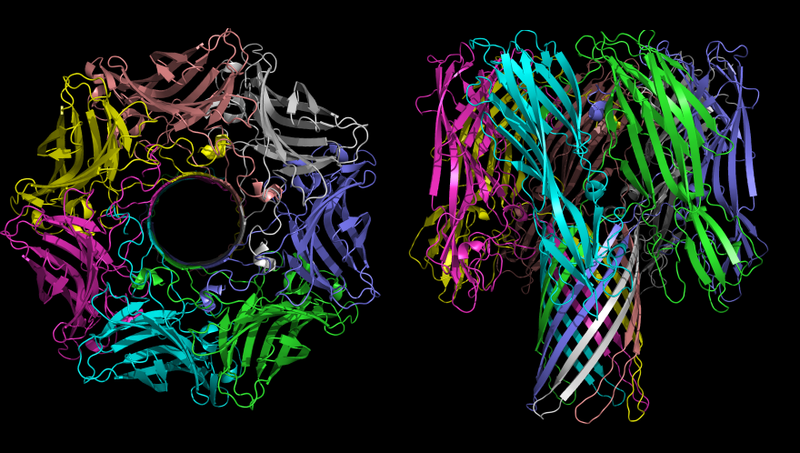
The three-dimensional structure of the oligomer α-hemolysin - toxin bacteria Staphylococcus aureus. The picture on the left is clearly visible time.
The main function of α-hemolysin in the pathogenic process is the creation of uncontrolled channels in the cell membrane, which, depending on the surrounding conditions, can pass through monovalent ions, calcium ions or ATP. As mentioned above, the ionic imbalance is extremely toxic, and the transport of ATP from the cell is harmful, since ATP is an indispensable element of almost all chemical processes of any cell.
As can be seen from the examples above, facilitated diffusion does not require energy. On the contrary, active transport is the absorption or release of something by the cell with the expenditure of energy for each transfer event. This can be the transport of large polar molecules or ions, and the transport of ions can be carried out both along the concentration gradient and against it. Of course, active transport is a controlled process in which a whole set of intracellular and transmembrane proteins is involved. Thus, first, the cell needs to expend resources to synthesize all these proteins, and then once again spend energy on the use of this transport system.
An example of active transport is the system of energy-dependent transport of hydrophobic and amphiphilic compounds from a cell back to the environment (efflux).

The system of active transport of the antibiotic from the bacterial cell to the outside, consisting of three proteins forming a single transmembrane complex. The figure reflects the fact that the efflux transporter (violet) protein can “catch” drug molecules both directly in the cytoplasm and in the membrane.
This system is of particular interest because it is the cause of multiresistance of some bacteria to antibiotics: the cell gets rid of them before they can seriously harm it. Moreover, more than half of cases of Pseduomonas aeruginosa Pseudomonas aeruginosa multiresistance are associated with active transport of antibiotics. More importantly, active efflux systems of “new” antibiotics have already been discovered, which are used against bacteria resistant to widely used antibacterial drugs. For example, the drug Linezolid used for the ineffectiveness of classical antibiotics is thrown back by efflux system based on AcrAB and TolC proteins. Another interesting fact is that a smart bacterium does not waste forces on large-scale protein synthesis of efflux systems in situations where they are not needed. But as soon as substances harmful to the cell appear, the synthesis is rapidly activated and the bacterium begins to actively resist. By the way, efflux is not the only mechanism for protecting bacteria against antibiotics, I will write an article about other methods too.
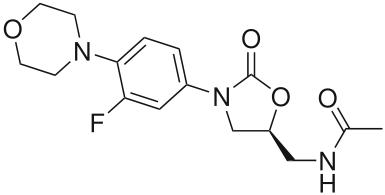
The structure of linezolid is an antibiotic used against resistant strains of gram-positive bacteria.
So, we have examined the mechanisms of transmembrane transport of compounds that cannot penetrate this barrier without assistance. Under natural conditions, DNA is transported to the bacterium only through active transport through the cooperation of a whole spectrum of both intracellular and transmembrane proteins. The non-induced ability of a cell to absorb DNA from the outside is called "natural competence."
The most substantiated hypothesis of the occurrence of the natural competence of bacteria is the assumption that this mechanism allows bacteria to survive in the conditions that are extreme for them (the conditions that are extreme for a cell are called "stress"). The fact is that stress often leads to damage to the “chromosomal” DNA of the bacterium, and if the cell wants to survive, then it needs to somehow “repair” its “chromosome” (this “repair” is called “repair”). At the same time, it is known that if a DNA sequence is introduced into a cell, which is similar to some part of the cell's own DNA, then it will most likely integrate this “new” piece into its genome. What happens under stressful conditions? Some cells die, becoming DNA donors for those of their fellows who are still fighting for life. Indeed, within the same bacterial culture it is very likely that the genomes of the representatives are very similar, therefore, the surviving bacteria can use the DNA of the dead relatives for the repair of their genome.
At the same time, under comfortable conditions in cell culture, only a part of them possess natural competence, and the proportion of competent cells of their total number is greatest then, then the culture is in a certain growth phase — the log phase.

A typical graph of the number of bacteria in culture from time to time. The log phase is a growth phase, followed by a plateau and a extinction stage, in which cells no longer have enough nutrients and toxins accumulate.
Of course, the researcher would like to make the transfer of the plasmid vector into the cell as efficient as possible: the more efficient the transformation process, the less plasmid vector is needed in order to guarantee good results. Therefore, under laboratory conditions, they usually do not rely on the cell itself, but instead create cells with artificial competence.
There are two main methods for creating artificial competence of bacteria: treatment of cell culture with bivalent ions, followed by brief heating and so-called electroporation.
As a rule, the laboratory has a refrigerator with a temperature of about -80 degrees Celsius (sometimes we, by analogy with “xeroxes”, are called “kelvinators” in honor of one of the manufacturers), clogged with test tubes with cells that are in log phase. This is done in order not to waste time on their creation each time they are needed (and it takes quite a lot of time, it will not be possible to make them faster than in a day). And so that during storage cells do not die due to freezing cryoprotectants are added to the medium: glycerol, propylene glycol or dimethyl sulfoxide.
Laboratory refrigerator in the view of the author of the picture.
When a researcher needs to transform a particular strain, he pulls the cells out of the kelvinator and places the tube in the ice. Then bivalent cations and the plasmid vector we need are added to the cells in the growth phase log- phase. The test tube should not leave the ice. Calcium chloride (CaCl2) is a typical donor of such cations, in this case Ca2 + is our bivalent cation. There is no unambiguous explanation of how Ca2 + helps DNA penetrate inside the bacteria:
It is possible that both hypotheses are true.
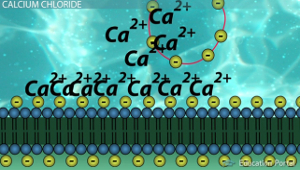
The proposed mechanism for the participation of Ca2 + ions during transformation. First, the ions join negatively charged groups of DNA (yellow circles) and polysaccharides anchored in the membrane (yellow circles). Then, due to the ion, a kind of bridge is formed: “polysaccharide (-)” - “Ca (2+)” - “DNA (-)”, which fixes the DNA on the cell surface.
After the bacteria are incubated at near-zero temperature, a so-called heat shock (heat shock) is produced - a short-term heating of the culture to 42 degrees. Typically, the duration of thermal treatment is 30-45 seconds, after which the tube with bacteria returns to the ice bath (with a longer heat shock, the cells may die). Possible reasons for the effectiveness of heat shock are the same mechanisms: high temperature can damage DNA and proteins (this induces competence), and can also create “holes” in the cell membrane, through which plasmid vectors attached to the membrane penetrate inside.
Then the cells are sown on Petri dishes with selective medium and colonies grow on them.
The basis of the electroporation method is the fact that the electric field can damage the cell wall. That is, literally, electroporation is the creation of pores by an electric field.
First, bacteria and a plasmid vector are placed in a special test tube (cuvette). Then the cuvette is placed in a special device, which for a fraction of a second creates a potential difference of 0.1-10 kV, which in turn leads to reversible damage to the membranes and the vectors can freely penetrate into the cells. After some time, the bacterial culture is seeded on Petri dishes with selective medium.
An important requirement for the liquid medium in which the cells and the vector are located at the time of electroporation is a low concentration of ions: otherwise a short circuit is possible.
Each colony on the plate is the breeding descendants of one single bacterium (that is, if we see 100 colonies on the plate, this means that initially there were 100 viable bacteria, which then began to divide). After the colonies appear on the plates, the researcher chooses one of the colonies and transfers the cells from the colony to a new selective liquid nutrient medium. After the new cell culture "grows up" it is again planted on a new cup with the same selective nutrient medium. As a result, we get a cup on which only descendants of bacteria from a single colony grow (that is, in fact, all of them are clones of a single bacterium). And only these cells will already be used in the future to get the protein we need in large volumes of liquid selective nutrient medium.
Well, let's sum up the intermediate results:
The next step is to isolate the protein from the resulting biomass in as pure a form as possible. But that's another story.

So, we have a plasmid vector on hand and there is a bacterial strain suitable for this vector, it remains to add the vector to the bacterium.
The previous article mentioned the work of Frederick Griffith , which clearly demonstrates the fact that DNA can spontaneously penetrate bacterial cells. The ability of a bacterial cell to absorb the environmentally located DNA molecule is called “competence” (which gives rise to a lot of jokes about the fact that even single-celled in vitro is competent, and your laboratory colleague is not). If absorption occurs not under the influence of some artificially created conditions, then we are talking about “natural competence”. How does the similar DNA transport inside the bacteria occur?
1) The structure of the cell wall of bacteria
To begin, consider briefly the structure of the shell of gram-positive and gram-negative bacteria.
The shell of a gram-negative bacterium consists of two lipid bilayers: the outer membrane and the inner membrane (the inner one is also called the plasmatic membrane). Between them there is a periplasmic space filled with important proteins and peptidoglycan.
')

On the left - the structure of the shell of gram-negative bacteria. The colored things inside the membranes are membrane proteins, and the chains sticking outward are lipopolysaccharides. On the right - the structure of the most gram-negative bacteria.
A peptidoglycan is a heteropolymer consisting of covalently crosslinked N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). It turns out the chain ....- NAG-NAM-NAG-NAM-NAG- .... “Hetero” in the word “heteropolymer” means that the polymer consists not of the same elements (as polyethylene), but of two different. The synthesizing peptidoglycan transpeptidase enzyme is a target for β-lactam antibiotics (penicillin antibiotics and cephalosporins): they suppress its activity, as a result of which the peptidoglycan is not sufficient for both daughter cells. The peptidoglycan layer is also a target for the lysozyme enzyme: it breaks the covalent bond between NAG and NAM. In medicine, lysozyme is used as an antiseptic, for example, it is an active component of some drugs for treating sore throat, and in the food industry it can be used as a preservative (food additive E1105 is lysozyme).

Peptidoglycan. The figure shows the part corresponding to one NAG (it is on the left) and one NAM (it is on the right), which are interconnected through an oxygen atom. (For those who do not like such liberties in chemistry: this "bond through an oxygen atom" is called β- (1,4) -glycosidic).
The shell of gram-positive bacteria consists only of the inner (plasma) membrane, a very thin periplasmic space and an outer, thicker peptide layer than the gram-negative bacteria.
Such obstacles that stand in the way of any compound into the bacterial cytoplasm are insurmountable for everything except water, as well as small non-polar and hydrophobic molecules (molecular oxygen and nitrogen, carbon dioxide and others). The penetration of these compounds through the envelope is called “passive transport” (“simple diffusion”); it always occurs along a concentration gradient. Non-polar molecules are able to freely pass through the membranes because the internal volume of the membranes is also non-polar (inside the membrane they consist of hydrophobic fatty acid residues), and non-polar compounds dissolve well in non-polar solvents (alcohol, acetone, fatty acids).

A comic picture in which the hydrophobicity (hydrophobia) of fatty acid residues inside a two-layer membrane is played up.
In turn, large polar molecules (amino acids, monosaccharides, nucleotides), charged particles (ions) and macromolecules (DNA, proteins) cannot penetrate through the cell membrane just like that, since they are well soluble only in polar solvents (for example, in water) . Then how do DNA molecules get inside the bacterial cell?
2) Mechanisms of induced transport of compounds into cells from the external environment
First of all, we have on our hands the obvious fact that the bacterium cannot live only on water, oxygen and carbon dioxide, it needs many other compounds for its vital activity. So there are ways to deliver these compounds into the cell. Such methods are “facilitated diffusion” and “active transport”.
2.1) Light diffusion
An example of facilitated diffusion (although this particular case is contrary to bacteria for bacteria) is the transport of potassium ions through the membrane of a bacterial cell in combination with valinomycin, an antibiotic that is synthesized by some fungi. Free valinomycin cannot penetrate inside the cell, since polar groups are exposed to it outside. Potassium ion is also "way closed."

The sculpture depicting the three-dimensional structure of the complex of valinomycin and potassium ion (ion depicted as a ball in the center). Installed in front of the entrance to the Institute of Bioorganic Chemistry of the Russian Academy of Sciences named after M. Shemyakin and Yu. A. Ovchinnikov (Moscow), which established the mechanism of antibacterial activity of valinomycin and the three-dimensional structure of its complex with potassium ion.
But after forming a complex of valinomycin with a potassium ion, it changes its three-dimensional structure in such a way that its entire outer surface becomes hydrophobic (non-polar), and all hydrophilic groups are hidden inside. At the same time, the potassium ion is hidden inside the molecule of valinomycin, therefore the complex “valinomycin + potassium ion” can penetrate through the membrane. The complex can break up to free valinomycin and an ion at any time, both inside the cell and outside, but in general, facilitated diffusion of ions leads to the equalization of their concentration both in the intracellular and extracellular space, and the cell controls the ion transport for a reason: high concentration destructive. This is the basis of the antibacterial effect of valinomycin.

Schematic representation of the fungal antibiotic valinomycin, which formed a complex with potassium ion.
Another example is uncontrolled pores, which are large transmembrane proteins that allow certain substances to pass unhindered through the membrane according to a gradient. An example of such a transmembrane pore is the α-toxin of Staphylococcus aureus Staphylococcus aureus (another name for the toxin: α-hemolysin. It consists of “hemo” and “lysine” parts. “Hemo” means red blood cells are the target of the toxin; red blood cells; lysine - from the word "lysis", that is, "cell death")

The three-dimensional structure of the oligomer α-hemolysin - toxin bacteria Staphylococcus aureus. The picture on the left is clearly visible time.
The main function of α-hemolysin in the pathogenic process is the creation of uncontrolled channels in the cell membrane, which, depending on the surrounding conditions, can pass through monovalent ions, calcium ions or ATP. As mentioned above, the ionic imbalance is extremely toxic, and the transport of ATP from the cell is harmful, since ATP is an indispensable element of almost all chemical processes of any cell.
2.2) Active transport
As can be seen from the examples above, facilitated diffusion does not require energy. On the contrary, active transport is the absorption or release of something by the cell with the expenditure of energy for each transfer event. This can be the transport of large polar molecules or ions, and the transport of ions can be carried out both along the concentration gradient and against it. Of course, active transport is a controlled process in which a whole set of intracellular and transmembrane proteins is involved. Thus, first, the cell needs to expend resources to synthesize all these proteins, and then once again spend energy on the use of this transport system.
An example of active transport is the system of energy-dependent transport of hydrophobic and amphiphilic compounds from a cell back to the environment (efflux).

The system of active transport of the antibiotic from the bacterial cell to the outside, consisting of three proteins forming a single transmembrane complex. The figure reflects the fact that the efflux transporter (violet) protein can “catch” drug molecules both directly in the cytoplasm and in the membrane.
This system is of particular interest because it is the cause of multiresistance of some bacteria to antibiotics: the cell gets rid of them before they can seriously harm it. Moreover, more than half of cases of Pseduomonas aeruginosa Pseudomonas aeruginosa multiresistance are associated with active transport of antibiotics. More importantly, active efflux systems of “new” antibiotics have already been discovered, which are used against bacteria resistant to widely used antibacterial drugs. For example, the drug Linezolid used for the ineffectiveness of classical antibiotics is thrown back by efflux system based on AcrAB and TolC proteins. Another interesting fact is that a smart bacterium does not waste forces on large-scale protein synthesis of efflux systems in situations where they are not needed. But as soon as substances harmful to the cell appear, the synthesis is rapidly activated and the bacterium begins to actively resist. By the way, efflux is not the only mechanism for protecting bacteria against antibiotics, I will write an article about other methods too.

The structure of linezolid is an antibiotic used against resistant strains of gram-positive bacteria.
3) Bacterial cell competence
So, we have examined the mechanisms of transmembrane transport of compounds that cannot penetrate this barrier without assistance. Under natural conditions, DNA is transported to the bacterium only through active transport through the cooperation of a whole spectrum of both intracellular and transmembrane proteins. The non-induced ability of a cell to absorb DNA from the outside is called "natural competence."
3.1) Natural Bacterial Cell Competence
The most substantiated hypothesis of the occurrence of the natural competence of bacteria is the assumption that this mechanism allows bacteria to survive in the conditions that are extreme for them (the conditions that are extreme for a cell are called "stress"). The fact is that stress often leads to damage to the “chromosomal” DNA of the bacterium, and if the cell wants to survive, then it needs to somehow “repair” its “chromosome” (this “repair” is called “repair”). At the same time, it is known that if a DNA sequence is introduced into a cell, which is similar to some part of the cell's own DNA, then it will most likely integrate this “new” piece into its genome. What happens under stressful conditions? Some cells die, becoming DNA donors for those of their fellows who are still fighting for life. Indeed, within the same bacterial culture it is very likely that the genomes of the representatives are very similar, therefore, the surviving bacteria can use the DNA of the dead relatives for the repair of their genome.
At the same time, under comfortable conditions in cell culture, only a part of them possess natural competence, and the proportion of competent cells of their total number is greatest then, then the culture is in a certain growth phase — the log phase.

A typical graph of the number of bacteria in culture from time to time. The log phase is a growth phase, followed by a plateau and a extinction stage, in which cells no longer have enough nutrients and toxins accumulate.
Of course, the researcher would like to make the transfer of the plasmid vector into the cell as efficient as possible: the more efficient the transformation process, the less plasmid vector is needed in order to guarantee good results. Therefore, under laboratory conditions, they usually do not rely on the cell itself, but instead create cells with artificial competence.
3.2) Artificial Competence of Bacterial Cells
There are two main methods for creating artificial competence of bacteria: treatment of cell culture with bivalent ions, followed by brief heating and so-called electroporation.
3.2.1) Treatment of cell culture with bivalent ions, followed by brief heating.
As a rule, the laboratory has a refrigerator with a temperature of about -80 degrees Celsius (sometimes we, by analogy with “xeroxes”, are called “kelvinators” in honor of one of the manufacturers), clogged with test tubes with cells that are in log phase. This is done in order not to waste time on their creation each time they are needed (and it takes quite a lot of time, it will not be possible to make them faster than in a day). And so that during storage cells do not die due to freezing cryoprotectants are added to the medium: glycerol, propylene glycol or dimethyl sulfoxide.

Laboratory refrigerator in the view of the author of the picture.
When a researcher needs to transform a particular strain, he pulls the cells out of the kelvinator and places the tube in the ice. Then bivalent cations and the plasmid vector we need are added to the cells in the growth phase log- phase. The test tube should not leave the ice. Calcium chloride (CaCl2) is a typical donor of such cations, in this case Ca2 + is our bivalent cation. There is no unambiguous explanation of how Ca2 + helps DNA penetrate inside the bacteria:
- Some sources claim that high concentrations of Ca2 + cause gaps in the cell wall and DNA damage, and earlier we found that DNA damage is a signal for a cell to become competent;
- According to other sources, these ions serve as a “bridge” that attaches the plasmid vector to the cell. The fact is that positively charged ions are simultaneously attached to negatively charged groups of polysaccharides on the outer membrane of bacteria, and to negatively charged groups of plasmid DNA. If there were no ions in the medium, then the DNA would be repelled from the cell, and this is completely useless to the researcher.
It is possible that both hypotheses are true.

The proposed mechanism for the participation of Ca2 + ions during transformation. First, the ions join negatively charged groups of DNA (yellow circles) and polysaccharides anchored in the membrane (yellow circles). Then, due to the ion, a kind of bridge is formed: “polysaccharide (-)” - “Ca (2+)” - “DNA (-)”, which fixes the DNA on the cell surface.
After the bacteria are incubated at near-zero temperature, a so-called heat shock (heat shock) is produced - a short-term heating of the culture to 42 degrees. Typically, the duration of thermal treatment is 30-45 seconds, after which the tube with bacteria returns to the ice bath (with a longer heat shock, the cells may die). Possible reasons for the effectiveness of heat shock are the same mechanisms: high temperature can damage DNA and proteins (this induces competence), and can also create “holes” in the cell membrane, through which plasmid vectors attached to the membrane penetrate inside.
Then the cells are sown on Petri dishes with selective medium and colonies grow on them.
3.2.2) Electroporation
The basis of the electroporation method is the fact that the electric field can damage the cell wall. That is, literally, electroporation is the creation of pores by an electric field.
First, bacteria and a plasmid vector are placed in a special test tube (cuvette). Then the cuvette is placed in a special device, which for a fraction of a second creates a potential difference of 0.1-10 kV, which in turn leads to reversible damage to the membranes and the vectors can freely penetrate into the cells. After some time, the bacterial culture is seeded on Petri dishes with selective medium.
An important requirement for the liquid medium in which the cells and the vector are located at the time of electroporation is a low concentration of ions: otherwise a short circuit is possible.
Afterword
Each colony on the plate is the breeding descendants of one single bacterium (that is, if we see 100 colonies on the plate, this means that initially there were 100 viable bacteria, which then began to divide). After the colonies appear on the plates, the researcher chooses one of the colonies and transfers the cells from the colony to a new selective liquid nutrient medium. After the new cell culture "grows up" it is again planted on a new cup with the same selective nutrient medium. As a result, we get a cup on which only descendants of bacteria from a single colony grow (that is, in fact, all of them are clones of a single bacterium). And only these cells will already be used in the future to get the protein we need in large volumes of liquid selective nutrient medium.
Well, let's sum up the intermediate results:
- We synthesized the gene we needed;
- Inserted it into a suitable plasmid vector;
- A suitable producer strain was selected and transformed;
- Got a clone culture;
- Finally, we transferred a part of these clones to a large volume of the nutrient medium in order to get the protein we need in a large volume.
The next step is to isolate the protein from the resulting biomass in as pure a form as possible. But that's another story.
Source: https://habr.com/ru/post/402069/
All Articles