Researchers have developed a technology for rapid defrosting of cryopreserved tissue.

In some science fiction works mentioned one of the possibilities of traveling in space for long distances. A similar way is suggested to be used for “time travel”: a person is frozen, and after a certain time the system starts defrosting, and the “traveler” wakes up in a distant (or not so) future. There is a similar system of “sleep” in reality - some companies offer hopelessly sick people to undergo a cryopreservation procedure so that in the future, when scientists find a way to treat the once incurable diseases, a person is unfrozen and cured.
Unfortunately, fantasy is still fantasy, and people who have used the services of these companies will hardly ever be unfrozen and cured - too much damage is done to the cells of the tissue during freezing, and even more damage during the reverse process, heating. For modern specialists, the problem is not cryopreservation, but defrosting. However, it is now known about the technology that allows defrosting large fragments of tissue without damaging the cellular structure. This, of course, is not a cryon for a traveler to a distant star, but a great option for modern medicine. This method makes it possible to store organs for transplantation for a long time.
In general, cryopreservation itself is not a new method. The cryopreservation of cell cultures and tissues, the so-called embryos, has been developed and successfully applied for quite a long time. But until recently, reliable methods of “deep freezing” of individual organs did not exist. There are only a few cases of transplantation of frozen and then successfully defrosted organs, since it is usually a matter of preserving in a frozen organ of individual sections of living tissue that survive in a foreign body and gradually restore the functionality of the organ.
')
Working temperatures of cryopreservation are about -196 ° C. Capsules with living tissue are placed in liquid nitrogen, which allows you to completely stop the biochemical processes in the cells, including metabolism and energy with the external environment. Ideally, frozen tissue can last for a very long time, and if the sample is small in volume, it can be repaired.
The work of a team of scientists from Minnesota, USA, promises to change the scope of transplantation. Specialists announced the development of a new technology that allows them to freeze tissue samples and organs (in the future) without damaging the cells. “For the first time, someone was able to scale the cryopreservation method on biological systems, showing successful rapid defrosting of the stored tissue without damaging it,” said one of the University of Minnesota experts John Bischoff.
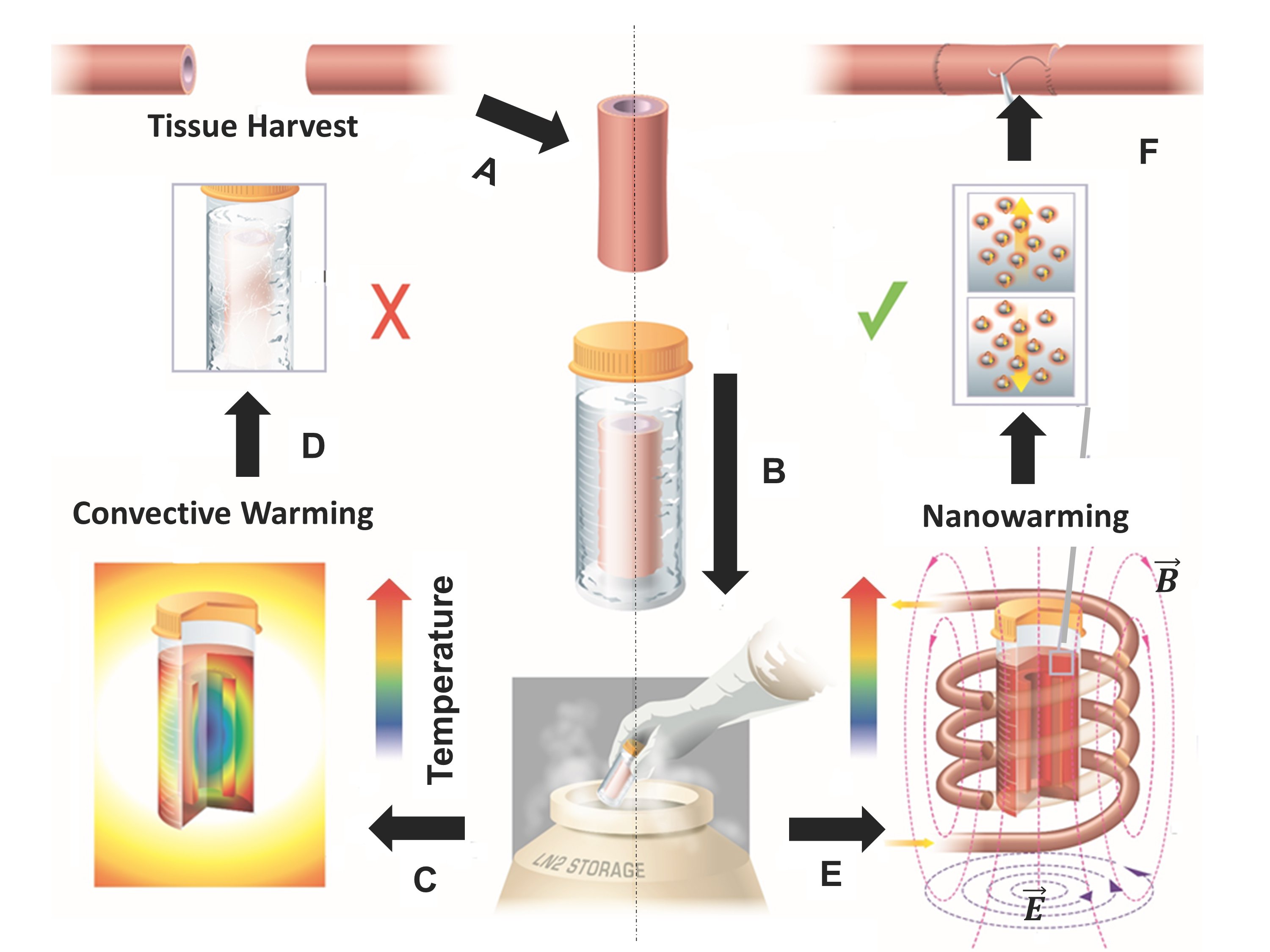
Instead of using convection generally used to defrost tissues, the authors of the project used nanoparticles to heat tissues, with an equal temperature rise for all sites. Moreover, the temperature rise during defrosting is very rapid - more than a hundred degrees per minute. As a result, ice crystals are not formed, which damage the cells.
Source: Manuchehrabadi et al., Science Translational Medicine (2017)
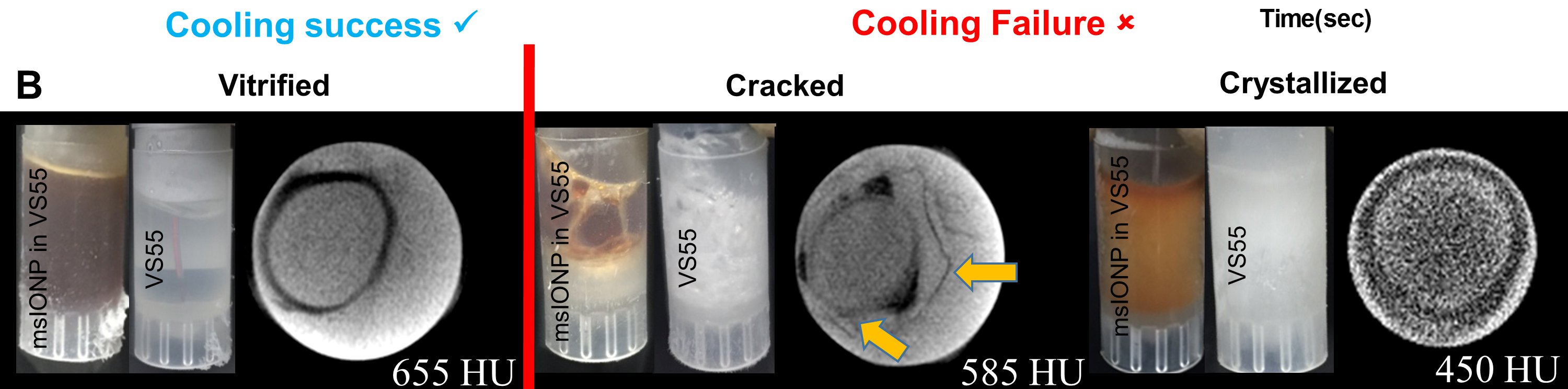
For this purpose, nanoparticles of iron oxide coated with silica are used. They are heated by the induced magnetic field. So far, the volumes of tissue elements thus preserved are small - from 1 to 50 ml. In experiments, the authors test their technology by dividing samples into experimental and control groups. The experimental group of frozen tissues is heated as described above. Control - in the usual way, using convection. Scientists have already conducted many experiments, but samples from the experimental group have never suffered, unlike samples from the control group.
On the left is a tissue thawed with the help of a new technique. On the right, after the red line - the fabric thawed in the traditional way. Source: Manuchehrabadi et al., Science Translational Medicine (2017)
After the restoration of the normal temperature regime, scientists remove the nanoparticles from the sample by leaching.
The technique was also tested when the system was heated with a volume of 80 ml, although this time without tissue. But, as it turned out, the heating rate is the same as in the case of systems with smaller volumes, which can be considered one of the proofs of the scalability of the technology. "In short, the nano-heating works with samples of 1 ml, 50 ml and can be scaled for 80 ml of systems," the authors of the article said . According to scientists, in the future, heating using nanoparticles can be applied to samples of much larger volume, up to 1 liter or more.
In this case, the nanoparticles will have to enter into tissue and organ samples by injection. The team has not yet tried its methodology for larger samples, although it plans to do so in the near future.
The main damaging factors during the freezing of living tissue are the formation of intracellular ice and cell dehydration. If the cooling is at high speed, ice crystals form inside the cell. And this, in turn, entails an increase in the internal volume of such structures as the Golgi apparatus, mitochondria, the endoplasmic reticulum, lysosomes, and the cytoplasmic membrane with their subsequent destruction. As for dehydration, when cooled, the cell loses about 80-90% of water, which leads to the destruction of hydrated complexes with macromolecules, after which the thawed cell cannot function normally.
Now scientists have learned to freeze tissue samples without damage. But defrosting is a problem that is being solved by scientists from many countries. If this issue can be resolved, doctors will be able to store frozen organs and tissues for a long time.
Source: https://habr.com/ru/post/402061/
All Articles