Rocket Fuel Saga

Preface.
And it will not be, that would not spam, everything is described in the "afterword". Be sure to read it so that there is no dissonance with earlier comments.The article had to be remade on 03/01/2017 and it differs from the original one.
Achtung! You should not consider this article as a kind of scientific work or a claim to the Nobel Prize.
Especially:
"... And there is nothing new under the sun"(Ecclesiastes 1: 9).
About fuel, rocket engines, rocket engines have been written, written and will be written.
One of the first work on fuel rocket engines can be considered the book VP Glushko "Liquid fuel for jet engines", published in 1936.

')
For me, the topic seemed interesting, related to my former specialty and study at the university, even more so as my younger son “dragged” her, “let's make the chef what thread we will start, and if we are lazy, then we will figure it out .” Apparently Lynn Lin Industrial " do not give rest.
So you want to properly blow up your rocket engine.
"Consider" will be together under strict parental control.
"The key to start" ... "Let's go"! (Yu.A. Gagarin & S.P. Korolev)

Whatever type of RD (scheme, nature of the process) is not used in rocket technology, its intended purpose: the creation of thrust (force), by converting the initial energy stored in the RT into kinetic energy (Ec) of the jet stream of the working fluid.
Ek jet of the jet in the RD will convert different types of energy (chemical, nuclear, electric).
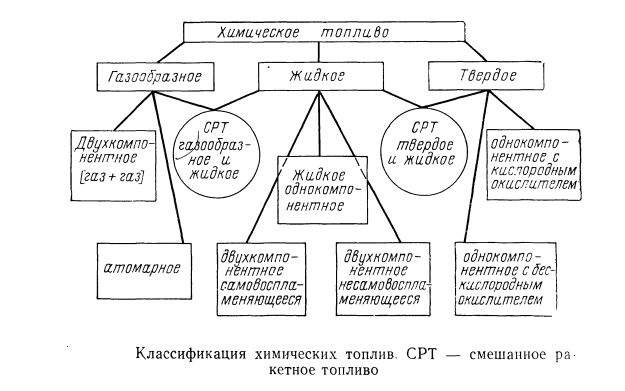
For chemical engines, fuel can be divided by phase state: gaseous, liquid, solid, mixed.
Part number 1 for fuel rocket engine or liquid rocket fuel.
Classification of chemical fuels for rocket engines (common):
Terms and abbreviations
LRE (RD) -liquid rocket engine.
The rocket engine is the resultant of the reactive force of the rocket engine and the environmental pressure forces acting on its external surfaces, with the exception of external aerodynamic drag forces. There are traction on the ground (at sea level) and in the void.
The specific impulse of the LRE (specific impulse of the LRE) is the ratio of the thrust of the LRE to the mass fuel consumption of the LRE. Similarly, the specific impulse of the LRE is maximal in the vacuum and accordingly decreases in the presence of ambient pressure.
LRE specific gravity is the ratio of the mass of the molten LRE to its greatest load on the main mode, and the mass of the molten LRE is determined by the mass of the LRE (mass of the LRE design) and fuel components that fill its pipelines and units during operation.
Type . Typically, each remote control is designed for a well-defined fuel, and the specific parameters of the liquid-propellant rocket engine and remote control and the effectiveness of their use as part of a rocket (or aircraft) largely depend on it.
The operation time of the LRE is the time from the first team to launch the LRE to the first team to shut it down. For multiple inclusion LREs, the operating time is equal to the total LRE operating time corresponding to all work cycles.
HRT-chemical rocket fuel.
HRT-liquid rocket fuel.
THA-turbopump unit.
KS - the combustion chamber.
Specific impulse (Iud) .
Jet thrust (P or Fp) .
KM construction materials.
DU-propulsion system.
O-oxidizer.
G-fuel.
Rocket fuel (TC, so as not to be confused with RT, see below) is a substance that undergoes chemical, nuclear or thermoelectric reactions in a rocket engine, to create its thrust.
The working body (RT) is a substance with which various physical and chemical transformations take place inside the RD, which constitute its working process.
The stoichiometric ratio of the components of the fuel (Km0) ( more-click ) is the ratio of the mass of oxidizer to the mass of fuel during stoichiometric reactions.
The fuel composition is combustible and non-combustible parts (in general).
Types of fuels (in general).
In general, a chemical source of thermal energy for RD can be considered the chemical reaction of the components of the RT.
I'll start broadcasting with Km0. This is a very important relationship for RD: fuel can burn differently in RD (a chemical reaction in RD is not the usual burning of firewood in a fireplace , where air oxygen acts as an oxidizer). Combustion (more precisely, oxidation) of fuel in the chamber of a rocket engine is, first of all, a chemical oxidation reaction with the release of heat. And the course of chemical reactions essentially depends on how many substances (their ratio) reacts.
How to fall asleep on the protection of the course project, exam or test. / Dmitry Zavistovsky
The value of Km0 depends on the valence that chemical elements may exhibit in the theoretical form of the chemical reaction equation. Example for : + UDMH .
An important parameter is the coefficient of excess oxidant (symbolized Greek "α" with an index of "approx.") And the mass ratio of the components of the Km.
Km = (dm. / Dt) / (dmg ../ dt), i.e. the ratio of the mass flow rate of the oxidizer to the mass flow of fuel. It is specific to each fuel. In the ideal case, is the stoichiometric ratio of oxidizer and fuel, i.e. shows how much kg of oxidizer is needed to oxidize 1 kg of fuel. However, the real values are different from the ideal. The ratio of real Km to the ideal is the coefficient of excess oxidant.

As a rule, αok. <= 1. And that's why. The dependences Tk (α.) And I Aud. (Α .) Are nonlinear and for many fuels the latter has a maximum at α. not with a stoichiometric ratio of components, i.e. max. Iud values are obtained with a slight decrease in the amount of oxidizing agent with respect to the stoichiometric one.
A little more patience, because I can not circumvent the concept: enthalpy . It is useful in the article and in everyday life.
Enthalpy is briefly energy. For the article are important two of its "incarnation":
Thermodynamic enthalpy - the amount of energy expended on the formation of a substance from the original chemical elements. For substances consisting of identical molecules (H2, O2, etc.), it is zero.
Enthalpy of combustion - makes sense only under the condition of a chemical reaction. In reference books one can find the values experimentally obtained under normal conditions. Most often for combustible it is full oxidation in the environment of oxygen, for oxidizing agents - oxidation of hydrogen by a given oxidizing agent. Moreover, the values can be both positive and negative, depending on the type of reaction.
“The sum of the thermodynamic enthalpy and enthalpy of combustion is called the total enthalpy of the substance. Actually, this value is used in the thermal calculation of the LRE chambers. ”
Requirements for :
-as a power source;
-as a power source;
-as to the substance that has to be used (at this level of technology development) to cool RD and THA, sometimes to pressurize tanks with RT, to provide it with volume (RN tanks), etc .;
-as to a substance outside the LRE, i.e. during storage, transportation, refueling, testing, environmental safety, etc.
Such gradation is relative conditional, but in principle reflects the essence.
I will name these requirements as follows: №1, №2, №3.
Someone can add to the list in the comments.
These requirements are the classic example of "Swan cancer and pike" , which "pull" the creators of RD in different directions:
# From the point of view of the energy source of LRE (# 1)

Those. need to get max. Id.
I will not continue to hammer heads with everything, in general:

With other important parameters for # 1, we are interested in R and T (with all indices).
It is necessary that: the molecular mass of the combustion products was minimal, the maximum was the specific heat content.
# From the point of view of the PH designer (№2):
TCs should have a maximum density, especially at the first stages of rockets, since they are the most voluminous and have the most powerful taxiways, with a large second consumption.
Obviously, this is not consistent with the requirement under No. 1

# From operational tasks are important (# 3):
- chemical stability of TC;
-easy refueling, storage, transportation and manufacturing;
-ecological safety (in the whole “field” of application), namely toxicity, cost of production and transportation, etc. and safety when operating the taxiway (explosive).
For details, see "The Saga of Rocket Fuels, the Other Side of the Medal . "


Of course this is only the tip of the iceberg. Additional requirements are also getting in here, because of which CONSENSUSES and COMPROMISES should be sought:
One of the components must have satisfactory (better excellent) properties of the cooler, since at this level of technology, it is necessary to cool the COP RD:

It is also required (as a rule) to use one of the components as a working fluid for a THA turbine:
For fuel components, “saturated vapor pressure is of great importance (roughly speaking, the pressure at which a liquid begins to boil at a given temperature). This parameter greatly influences the development of pumps and the weight of tanks. ”/ .. Fakas

An important factor is the aggressiveness of the TC to materials (KM) LRE and tanks for their storage.
If TCs are very “harmful” (like some people) then engineers have to spend money on a series of special measures to protect their structures from fuel.

- self-inflammability of the components of the fuel as a two-faced Janus : sometimes necessary, and sometimes it hurts. There is another nasty property: explosiveness
For many industries using missiles (military or deep space)
it is required that the fuel is chemically stable, and its storage, refueling (in general, everything that is called: logistics) and recycling do not cause a “headache” among operators and the environment.
An important parameter is the toxicity of combustion products. Now it is very relevant.

Cost of production: the load on the economy of the country, claiming the role of "space cab".
There are many of these requirements and, as a rule, they are antagonistic to each other.

Conclusion: the fuel or its components must have (or possess):
1. The greatest heat output, for maximum Iud.
2. The highest density, minimal toxicity, stability and low cost (in production, logistics and disposal).
3. The highest value of the gas constant or the lowest molecular weight of the combustion products, which will give Vmax flow and excellent specific impulse thrust.
4. A moderate combustion temperature (no more than 4500K), otherwise everything will burn or burn. Do not be explosive. Self-ignite under certain conditions.
5. Maximum rate of combustion. This will provide the minimum weight and volume of the COP.
6. The minimum ignition delay period, since smooth and reliable launch of the taxiway plays a significant role.
A whole bunch of problems and requirements: viscosity, melting and solidification, boiling temperature, evaporation, vapor pressure and latent heat of vaporization, etc. etc.
Compromises vividly manifest themselves in Id .: TK of high density (kerosene + LOX) are usually used on the lower stages of the PH, although they lose to the same L2 and LOX, which in turn are used on the upper stages of the PH ("Energy" 11K25) .

And again, an excellent pair of LH2 + LOX cannot be used for deep space or for long-term stay in orbit (Voyager-2, upper stage Briz-M, ISS, etc.)

Awesome moment of undocking the GOES-R meteorological satellite from the Centaur upper stage of the Atlas V 541 booster rocket ( GOES-R Spacecraft Separation )
Classification of HRT is most often based on the saturated vapor pressure or the triple point temperature , or more simply, the boiling point at normal pressure.
High-boiling components .
Chemical substance having a maximum operating temperature at which the saturated vapor pressure (I will refer to as RNP) in the missile tanks is significantly lower than the permissible pressure level in the tanks because of their structural strength.
Example:
kerosene, UDMH, nitric acid.
Accordingly, they are stored without special manipulations with cooling tanks.

I personally like the term “container” more. Although it is not entirely correct, but it is close to the everyday value. This is the so-called. long-life TC.
Low boiling components .
Here, the PNP is close to the maximum allowable pressure in the tanks (by the criterion of their strength). Storage in sealed tanks without special measures for cooling (and / or cooling) and returning the condensate is impossible. The same requirements (and problems) with the LRE fittings and refueling / discharge pipelines.
Example:
ammonia, propane, nitrogen tetroxide.

The Ministry of Defense of the Russian Federation (MO of the Russian Federation) considers all low-boiling components whose boiling points are below 298K under standard conditions.
In the range of temperatures of operation of rocket technology, low-boiling components are usually in a gaseous state. For the content of low-boiling components in a liquid state, special technological equipment is used.
Cryogenic components .
In fact, it is a subclass of low-boiling components.
Those. substances having a boiling point below 120K.
The cryogenic components include liquefied gases: oxygen, hydrogen, fluorine, etc. To reduce evaporative losses and increase the density, it is possible to use a cryogenic component in the bulk state, as a mixture of the solid and liquid phases of this component.
Special measures are required when refueling (cooling down of tanks and highways, heat-insulation of LRE fittings, etc.)


The temperature of their critical point is much lower than operational. Storage in sealed tanks PH is impossible or very difficult.
Typical representatives of oxygen and hydrogen in the liquid phase state.
Next, I will use the American style of their designations LOX and LH2, respectively. Or so the LCD and LW.
Our "handsome" RD-0120 (hydrogen-oxygen):

It is outside (valves, highways) completely filled with insulating material.
According to some experts, the production technology of RD-0120 has now been completely lost in the Russian Federation. However, on the basis of its technologies, an oxygen-hydrogen engine RD-0146 is created at the same enterprise.
When the components of the RT are found in the COP LRE (according to the "smart" react) they should be divided into:
self-igniting (STK), limited self-igniting (OSTK) and non-self-igniting TK (NTK).
STK: when the contact of the oxidizer and the fuel in the liquid state ignites (in the whole range of operating pressures and temperatures).
This greatly simplifies the ignition system of the taxiway, however, if the components meet outside the combustion chamber (leaks, accidents), then there will be a fire, or a big "women". Quenching difficult.

Example: N204 (nitric tetraxide) + MMG (monomethylhydrazine), N204 + N2H4 (hydrazine), N2O4 + UDMH and all fluorine-based fuels.
OSTK: special measures must be taken for ignition. Non-flammable fuels require an ignition system.

Example: kerosene + LOX or LH2 + LOX.
NTK: No comments here. It requires either a catalyst or a constant ignition (or temperature and / or pressure, etc.), or a third component.

Ideal for transportation, storage and leak-proof.
Another separation option is based on the level of energy characteristics of LRS:
* low-energy (with a relatively low specific impulse - one-component, etc.);
* medium-energy (with an average specific impulse— (02g) + kerosene, N204 + MMG, etc.);
* high-energy (with a high specific impulse: (02) g + (H2) G, (F2) g + (H2) g, etc.).
On toxicity and corrosivity of components distinguish :
* on non-toxic and non-corrosive-active components of fuel - (02) g, hydrocarbon combustible, etc .;
* on toxic and corrosion-active components of the fuel - MMG, UDMH and especially (F2) g.
According to the number of fuel components used, one-, two-, and three-component control systems are distinguished.
In one-component remote control, which most often use pressure feed.
At the initial stage of the development of auxiliary one-component remote control systems for satellites, spacecraft and spacecraft, highly concentrated (80 ... 95%) hydrogen peroxide was used as a one-component fuel.
At present, such auxiliary propulsion systems are used only in stage orientation systems of some Japanese PH.

For the remaining auxiliary one-component DUs, hydrogen peroxide is “displaced” by hydrazine, while an increase in the specific impulse by about 30% is ensured.
The widespread use of hydrazine in the LRE was largely promoted by the creation of highly reliable catalysts with a long resource, in particular the Shell 405 catalyst.
The most widely mankind uses two-component TCs, which have higher energy characteristics compared to single-component ones. But two-component fuel rocket engines are more complex in construction than single-component ones. Due to the presence of oxidizer tanks and fuel, a more complex piping system and the need to ensure the required ratio of fuel components (Km). In the remote control of the satellite, KK and KA often use not one but several tanks of oxidizer and fuel, which further complicates the pipeline system of a two-component remote control.

Three component RT in development. This is a real exotic.
Patent of the Russian Federation for a three-component rocket engine .
The scheme of this rocket engine .
Such LRE can be classified as multi-fuel.
A three-component fuel rocket engine (fluorine + hydrogen + lithium) was developed at OKB-456 .
Two-component fuels consist of an oxidizer and fuel.
LRE Bristol Siddeley BSSt.1 Stentor: a two-component LRE (H2O2 + kerosene)

Oxidizers.
Oxygen.
Chemical formula-O2 (oxygen, the American designation Oxygen-OX).
Liquid, rather than gaseous oxygen-liquid oxygen (LOX-briefly and everything is understandable) is used in the LRE.
Molecular mass (for the molecule) -32g / mol. For lovers of accuracy: atomic mass (molar mass) = 15.99903;
Density = 1,141 g / cm³
Boiling point = 90,188K (−182,96 ° C)
In terms of chemistry, the ideal oxidant.It was used in the first ballistic missiles of the FAA, its American and Soviet copies. But his boiling point did not suit the military. The required operating temperature range is from –55 ° C to + 55 ° C (long preparation time for launch, short time spent on combat duty).

Very low corrosivity. Production has long been mastered, the cost is small - less than $ 0.1 (in my opinion, a liter of milk is cheaper at times).
Disadvantages:

Cryogenic-necessary cooling down and constant refueling to compensate for losses before starting.

In the photo: the shutters of the protective devices of the kerosene filling station (ZU-2), 2 minutes before the end of the cyclogram when performing the operation CLOSE, the memory did not completely close due to icing. At the same time, due to icing, the signal for the TUA exit from the launcher did not pass. Start held the next day.
Difficult to use as a cooler of the COP and the nozzle rocket engine.

The unit-tanker RB liquid oxygen removed from the wheels and installed on the foundation.
Now everyone is exploring the possibility of using supercooled oxygen or oxygen in the slug-shaped state, in the form of a mixture of the solid and liquid phases of this component.
The view will be about the same as this beautiful ice slush in the cove to the right of Shamora:

Imagine: instead of H2O, imagine LCD (LOX).
Scrooping will increase the overall density of the oxidizer.
Example of cooling down (hypothermia) BR R-9A: for the first time, it was decided to use supercooled liquid oxygen as an oxidizer in a rocket, which reduced the total preparation time of the rocket for launch and increased its combat readiness.

Ozone -O3

=48 ..., =47,998 /
-188 °C (85,2 ) 1,59(7) /³
−195,7 ° (77,4 ) 1,73(2) /³
−197,2(2) ° (75,9 )
, .
, , , , (719 /). , , I. , (1,35 1,14 /³ ), (−112 °C −183 °C ).
O O2, 2 / 3·107 /2 (3 ), , - ( 24 % ). , -. , -170, -180, -191, I , .
Nitric acid -HNO3
Fluid condition at n.
Molar mass 63.012 g / mol (it does not matter that I use the molar mass or the molecular mass does not change the essence)
Density is 1.513 g / cm³
T. melt = - 41.59 ° C, T. kip. = 82.6 ° C
HNO3 , , , , , - . , . HNO3 – . “” “” , . - ! , – , , . ( «», ). , 0,5% () .
. (NO2). , , , 14% NO2. .
For almost 20 years we have been looking for a suitable container for nitric acid. It is very difficult to select construction materials for tanks, pipes, and LRE combustion chambers.
An option of an oxidizing agent that is chosen in the United States with 14% nitrogen dioxide. And our missilemen acted differently. It was necessary to catch up with the United States at any cost, so the oxidizers of Soviet brands - AK-20 and AK-27 - contained 20 and 27% of tetroxide.
An interesting fact: in the first Soviet rocket fighter BI-1, nitric acid and kerosene were used for flights.

Tanks and pipes had to be made from monel metal: an alloy of nickel and copper, it became a very popular construction material among the rocket men. Soviet rubles were almost 95% made of this alloy.

Disadvantages: tolerant "disgusting." Corrosive active. The specific impulse is not high enough. Currently in its pure form is almost never used.
Nitrogen tetraoxide -AT (N2O4)
Molar mass = 92,011 g / mol
Density = 1,443 g / cm³
"Took the baton" from nitric acid in military engines. Possesses saomovosplamenemost with hydrazine, UDMH. Low-boiling component, but can be stored for a long time when taking special measures.
Disadvantages: the same crap as HNO3, but with its own vagaries. May decompose to nitric oxide. Toxic.Low specific impulse. Often used and used oxidant AK-NN. It is a mixture of nitric acid and nitric tetroxide, sometimes referred to as "red fuming nitric acid." The numbers indicate the percentage of N2O4.

Basically, these oxidizing agents are used in the LRE for military use and the LRE spacecraft due to their properties: long-duration and self-igniting. Characteristic combustible for AT is UDMH and hydrazine.
Fluorine- F2
Atomic mass = 18,998403163 a. e. m. (g / mol)
Molar mass F2, 37.997 g / mol
Melting point = 53.53 K (−219.70 ° C)
Boiling point = 85.03 K (−188.12 ° C)
Density (for liquid phase), ρ = 1.5127 g / cm³
Fluorine chemistry began to develop since the 1930s, especially rapidly during the 2nd World War of 1939-45 and after it in connection with the needs of the nuclear industry and rocket technology. The name "Fluorine" (from the Greek. Phthoros - destruction, death), proposed by A. Ampere in 1810, is used only in Russian; in many countries, the name fluor is used .
This is an excellent oxidizing agent in terms of chemistry. Oxidizes both oxygen and water. Calculations show that the maximum theoretical Iud can be obtained on a pair of F2-Be (beryllium) order of 6000 m / s!
Super? Bummer, not “super” ...
Extremely corrosive, toxic, prone to explosions when in contact with oxidizing materials. Cryogenic Any product of combustion also has almost the same "sins": terribly corrosive and toxic.
Safety. Fluorine is toxic, its maximum permissible concentration in the air is about 2 · 10-4 mg / l, and the maximum permissible concentration at an exposure of no more than 1 hour is 1.5 · 10-3 mg / l.
LRE 8D21 using a pair of fluorine + ammonia gave a specific impulse at the level of 4000 m / s.
For a pair of F2 + H2 Iud = 4020 m / s is obtained!
Trouble: HF-hydrogen fluoride on the "exhaust".
Starting position after the launch of such an "energetic engine"?
A pool of liquid metals and other chemical elements dissolved in hydrofluoric acid!
H2 + 2F = 2HF, exists at room temperature as a dimer H2F2.
It is mixed with water in any respect with the formation of hydrofluoric (hydrofluoric) acid. And using it in the LRE spacecraft is not realistic due to storage difficulties.

All the same applies to other liquid halogens, for example, to chlorine.
Hydrogen fluoride LRE 25 tons to equip both stages of the rocket accelerator AKS Spiral was supposed to be developed in the OKB-456 VP Glushko on the basis of spent 10 tons of LRE with fluoroammic (F2 + NH3) fuel.
Hydrogen peroxide- H2O2.

It is mentioned by me above in one-component fuels.
Walter HWK 109-507: advantages in the simplicity of the design of the LRE. A vivid example of such a fuel is hydrogen peroxide.

Hydrogen peroxide for luxurious hair and 14 more secrets of use.

Alles: the list of less real oxidizers is over. Focusing on HCl O4 . As independent oxidants based on perchloric acid, they are of interest only: the monohydrate (H2O + ClO4) is a solid crystalline substance and the dihydrate (2NO + HClO4) is a dense viscous liquid. Perchloric acid (which is, by itself, unpromising), interest in the quality of an additive to oxidizing agents, which guarantees the reliability of auto-ignition of fuel.
Oxidizers can be classified as follows:

The final (more commonly used) list of oxidizers in conjunction with the real flammable ones:

Note: if you want to translate one version of a specific impulse into another, then you can use a simple formula: 1 m / s = 9.81 s.
In contrast, they are flammable.
Combustible
The main characteristics of two-component LRT at pc / pa = 7 / 0.1 MPa

According to the physico-chemical composition, they can be divided into several groups.
Hydrocarbon combustible.
Low molecular weight hydrocarbons.
Simple substances: atomic and molecular.
For this topic, so far, only hydrogen (Hydrogenium) is of practical interest.
Na, Mg, Al, Bi, He, Ar, N2, Br2, Si, Cl2, I2, etc. I will not discuss in this article.
Hydrazine fuels ("stinkers").
The search for the optimal fuel began with the development of LRE enthusiasts.
The first widely used fuel was alcohol (ethyl), which was used in the first
Soviet missiles R-1, R-2, R-5 ("inheritance" of the V-2) and at the Vergeltungswaffe-2 itself.

Rather, a solution of 75% ethanol (ethanol, ethyl alcohol, methyl carbinol, wine alcohol or alcohol, often simply “alcohol”) - a monohydric alcohol with the formula C2H5OH (empirical formula C2H6O), another option: CH3-CH2-OH
This fuel has two serious drawbacks that the military obviously did not like: low energy indicators and low resistance of personnel to the "poisoning" of such fuel.
Supporters of a healthy lifestyle (spirits) tried to use furfuryl alcohol — a poisonous, agile, transparent, sometimes yellowish (to dark brown), with time reddening liquid in air.

Chem. formula: C4H3OCH2OH, Rac. formula: C5H6O2. Disgusting swill.
Not intended for drinking.
Group of hydrocarbons.
Kerosene
Conditional formula C7,2107H13,2936
Combustible mixture of liquid hydrocarbons (from C8 to C15) with a boiling point in the range of 150-250 ° C, transparent, colorless (or slightly yellowish), slightly oily to the touch
density - from 0.78 to 0.85 g / cm³ (at a temperature of 20 ° );
viscosity - from 1.2 - 4.5 mm² / s (at a temperature of 20 ° C);
flash point - from 28 ° C to 72 ° C;
calorific value - 43 MJ / kg.
My opinion: it’s pointless to write about the exact molar mass

Kerosene is a mixture of various hydrocarbons, so there are terrible fractions (in chemical formula) and "smeared" boiling point.
Convenient high boiling fuel. It has been used for a long time and successfully all over the world in engines and in aviation. It is on it that the "Unions" are still flying. Low toxicity (I strongly recommend against drinking), stable. Still, kerosene is dangerous and harmful to health (ingestion).
But there are people who treat them nothing! Ministry of Health strongly opposed!
Soldier tales: well helps to get rid of nasty Pthirus pubis .
However, it also requires caution in handling: the accident of a passenger aircraft
Significant advantages: relatively inexpensive, mastered in production.
A kerosene-oxygen pair is ideal for the first stage. Its specific impulse on the earth is 3283 m / s, hollow 3475 m / s. Disadvantages. Relatively low density.

American Rocket Kerosene Rocket Propellant-1 or Refined Petroleum-1

Relatively cheap (was):
To increase the density of space exploration leaders, Sintine (USSR) and RJ-5 (USA) were developed.
Synthesis of sintin.
Kerosene tends to deposit tar in the highways and the cooling path, which has a negative effect on cooling.
At this his property is pedaled Mukhin, Velor @Co.
Kerosene engines are most mastered in the USSR.
A masterpiece of human mind and engineering is our “pearl” RD-170/171:

"Where do the best rocket engines in the world . "
Now, the more appropriate name for fuel based on kerosene has become the term HCG - “hydrocarbon fuel”, since from kerosene, which was burned in the safe kerosene lamps of I. Lukasevich and J. Zeha, the HCG used “went away” very far .
As an example: naphthyl .

Low molecular weight hydrocarbons
Methane -CH4
Molar mass: 16.04 g / mol
Density gas (0 ° C) 0.7168 kg / m³;
liquid (−164,6 ° C) 415 kg / m³
T. melt = - 182,49 ° C
T. Kip. = - 161,58 ° C
Everyone is now considered as a promising and cheap fuel, as an alternative to kerosene and hydrogen.
Chief Designer of NPO Energomash Vladimir Chvanov:
- The specific impulse of an engine for LNG is high, but this advantage is leveled by the fact that methane fuel has a lower density, so the total is a slight energy advantage. From a structural point of view, methane is attractive. To free up the cavities of the engine, you only need to go through a cycle of evaporation - that is, the engine is easier to get rid of residual products. Due to this, methane fuel is more acceptable from the point of view of creating a reusable engine and a reusable aircraft.
Inexpensive, common, stable, low toxic. Compared to hydrogen, it has a higher boiling point, and the specific impulse paired with oxygen is higher than that of kerosene: about 3250-3300 m / s on the ground.
Good cooler.
Disadvantages. Low density (twice lower than that of kerosene). In some modes of combustion, it can decompose with carbon in the solid phase, which can lead to a drop in the pulse due to two-phase flow and a sharp deterioration in the cooling mode in the chamber due to the deposition of soot on the walls of the CS. Recently, active NOR and R & D in the area of its application (along with propane and natural gas), even in the direction of modification, are already existing. LRE (in particular, such work was carried out on RD-0120 ).

Or as a “fresh” example of the American Raptor engine from Space X:

These fuels include propane and natural gas. Their main characteristics, like combustible ones, are close (with the exception of greater density and higher boiling point) to HCG. And there are the same problems with their use.
Separately among the combustible is positioned Hydrogen- H2 (Liquid: LH2).

The molar mass of hydrogen is 2,016 g / mol, or approximately 2 g / mol.
Density (with n. Y.) = 0.0000899 (at 273 K (0 ° C)) g / cm³
Melting point = 14.01 K (-259,14 ° C);
Boiling point = 20.28 K (-252.87 ° C);
The use of a LOX-LH2 pair is proposed by Tsiolkovsky, but implemented by others:

From the point of view of thermodynamics, H2 is an ideal working body both for the LRE itself and for the THA turbine. Excellent cooler, with both in the liquid and in the gaseous state. The latter fact allows us not to be particularly afraid of the boiling of hydrogen in the cooling path and to use the gasified hydrogen in this way to drive the THA.
This scheme is implemented in the Aerojet Rocketdyne RL-10-just smart (from an engineering point of view) engine:
Our analogue (even better, because younger): RD-0146 (D, DM) is a gas-free liquid-propellant rocket engine developed by the Chemical Engineering Design Bureau in Voronezh.

Especially effective with a nozzle nozzle from the material "Grauris".
High specific impulse-paired with oxygen 3835 m / s.

Of the actually used is the highest rate. These factors cause a keen interest in this fuel. Environmentally friendly, at the "outlet" in contact with O2: water (water vapor).
Distributed, almost unlimited reserves. Mastered in production. Nontoxic.
However, there are a lot of fly in the ointment in this barrel of honey.
1. Extremely low density. Everyone saw the huge hydrogen tanks of the PH Energia and the Space Shuttle Space Shuttle. Due to its low density, it is applicable (as a rule) in the upper stages of the LV.


In addition, low density poses a difficult task for pumps: hydrogen pumps are multi-stage in order to ensure the desired mass flow and not to cavitate.

For the same reason, you have to put a so-called. booster fuel pumping units (BNAG) immediately behind the intake device in the tanks, in order to facilitate the life of the main TNA.

Hydrogen pumps for optimum performance require significantly high speeds.
2. Low temperature. Cryogenic fuel. Before refueling, it is necessary to carry out many hours of cooling down (and / or overcooling) of tanks and the entire path. Bucky PH "Falocn 9FT" inside view:

More about "surprises":
"MATHEMATICAL MODELING OF HEAT AND MASS-EXCHANGE PROCESSES IN HYDROGEN SYSTEMS" HOP VA GordeevV.P. Firsov, A.P. Gnevashev, E.I. Postoyuk
FSUE "GKNPTs them. Mv Khrunicheva, Salyut; "Moscow Aviation Institute (State Technical University)
The paper describes the main mathematical models of heat and mass transfer processes in the tank and highways of oxygen oxygen-hydrogen block 12KRB. Anomalies in the supply of hydrogen in the LRE are revealed and their mathematical description is proposed. The models were worked out during bench and flight tests, which made it possible to predict the parameters of serial overclocking units of various modifications on their basis and make the necessary technical decisions to improve the pneumatic-hydraulic systems.
Low boiling point makes storage of this fuel difficult.
3. Liquid hydrogen has some gas properties:
Compressibility factor (pv / RT) at 273.15 K: 1.0006 (0.1013 MPa), 1.0124 (2.0266 MPa), 1.0644 (10.133 MPa), 1.134 (20.266 MPa), 1.277 (40.532 MPa) ;
Hydrogen can be in ortho and para states. Orthohydrogen (o-H2) has a parallel (of the same sign) orientation of nuclear spins. Para-hydrogen (p-H2) -anti-parallel.
At ordinary and high temperatures, H2 (normal hydrogen, n-H2) is a mixture of 75% ortho and 25% para-modifications, which can mutually transform into each other (ortho-para-transformation). When o-H2 is converted to p-H2, heat is released (1418 J / mol) .
This all imposes additional difficulties in the design of highways, LRE, THA, cyclograms of work, and especially pumps.
4. Hydrogen gas, faster than other gases, spreads in space, passes through small pores, at high temperatures it penetrates relatively easily through steel and other materials. H2G has a high thermal conductivity, equal at 273.15 K and 1013 hPa 0.1717 W / (m * K) (7.3 relative to air).
Hydrogen in the normal state at low temperatures is inactive, without heating it reacts only with F2 and in the light with l2. Hydrogen interacts with non-metals more actively than with metals. It reacts with oxygen almost irreversibly, forming water with the release of 285.75 MJ / mol of heat;
5. With alkaline and alkaline-earth metals, elements of group III, IV, V and VI of the periodic system, as well as with intermetallic compounds, hydrogen forms hydrides. Hydrogen reduces the oxides and halides of many metals to metals, unsaturated hydrocarbons to saturated (see Hydrogenation ).
Hydrogen gives up its electron very easily. In solution, it detaches as a proton from many compounds, causing their acidic properties. In aqueous solutions, H + forms a hydroxonium ion H3O with a water molecule. Being part of the molecules of various compounds, hydrogen tends to form with many electronegative elements (F, O, N, C, B, Cl, S, P) a hydrogen bond.
6. Fire resistance and explosiveness. You can not rassusolivat: everyone knows an explosive mixture.
A mixture of hydrogen with air explodes from the slightest spark in any concentration - from 5 to 95 percent.

So there is hydrogen and Gut (even Sehr Gut ), and at the same time “headache” (even severe headache).
The first law of dialectics: "Unity and struggle of opposites" / Georg Wilhelm Friedrich Hegel /
Impressive Space Shuttle Main Engine (SSME)?


Probably having seen it and considering its cost (the cost of putting 1 kg of PN into orbit), lawmakers and those who steer the US budget and NASA in particular ... decided "well, what for him".
I understand them, the Soyuz RN is cheaper and safer, and the use of the RD-180/181 removes many of the problems of American RN and significantly saves taxpayers money.

The best rocket engine is such an engine that you can produce / buy, while it will have a load in the required range (not too big or small) and will be so effective (specific impulse, pressure in the combustion chamber) that its price will not become too heavy for you.Philip Terekhov @ lozga
The most mastered hydrogen engines in the United States.
Now we are positioning at the 3-4 place in the “Hydrogen Club” (after Europe, Japan and China / India).
Separately mention solid and metallic hydrogen.

Solid hydrogen crystallizes in a hexagonal lattice (a = 0.378 nm, c = 0.6167 nm), in the nodes of which there are H2 molecules interconnected by weak intermolecular forces; density 86.67 kg / m³; C ° 4.618 J / (mol * K) at 13 K; dielectric. At pressures above 10,000 MPa, a phase transition is expected with the formation of a structure built of atoms and possessing metallic properties. The possibility of superconductivity "metallic hydrogen" is theoretically predicted.
Solid hydrogen is a solid state of hydrogen.
Melting point −259.2 ° C (14.16 K).
With a density of 0.08667 g / cm³ (at −262 ° C).
White snow-like mass, crystals of hexagonal syngony.
The Scottish chemist J. Dewar in 1899 for the first time received hydrogen in a solid state.
For this, he used a regenerative cooling machine based on the Joule-Thomson effect.

Trouble with him. It is constantly being lost: "Scientists have lost the world's only sample of metallic hydrogen . "
For some reason, it reminded me of the “nanotank of Chubais” . This nano-miracle can not find already 7 years or more.
Anamason, antimatter, metastable helium while I leave behind the scenes.
...
Hydrazine Fuels ("Stinkers")
Hydrazine-N2H4
A condition at NU. - colorless liquid
Molar mass = 32.05 g / mol
Density = 1.01 g / cm³
Very common fuel.
It is stored for a long time, and it is “loved” for it. It is widely used in remote control of spacecraft and ICBM, i.e. where timeliness is critical.

Whom embarrassed Iud in the dimension of H * s / kg I answer: this is a military designation.
Newton is a derived unit, based on Newton's second law, it is defined as a force that changes the speed of a body weighing 1 kg per 1 m / s in 1 second in the direction of the force. Thus, 1 N = 1 kg · m / s 2 .
Accordingly: 1 N * s / kg = 1 kg · m / s 2 * s / kg = m / s.
Mastered in production.

Disadvantages: toxic, smelly.
For humans, the degree of toxicity of hydrazine is not determined. According to S. Krop's calculations, a dangerous dangerous concentration is 0.4 mg / l. Ch. Comstock with employees believes that the maximum permissible concentration should not exceed 0.006 mg / l. According to later American data, this concentration at an 8-hour exposure is reduced to 0.0013 mg / l. It is important to note at the same time that the threshold of the olfactory sensation of hydrazine in humans significantly exceeds the indicated numbers and is equal to 0.014–0.030 mg / l. Essential in this regard is the fact that the characteristic smell of a number of hydrazino derivatives is felt only in the first minutes of contact with them. Subsequently, as a result of the adaptation of the organs of smell, this sensation disappears, and the person, not noticing it, can stay for a long time in an infected atmosphere containing toxic concentrations of the substance.
. , (). .
()-H2N-N(CH3)2
. :C2H8N2, . :(CH3)2NNH2
..-
=60,1 /
=0,79±0,01 /³
. - .
.

.
. .
... .

( -200).

, , , .

: . «». .

-.. -50, 50- . .
- Dassault Mirage III ( -) .
.

; ( = ·/ = ·/). ( 9,81 /²)
:
, -, - CH3NHNH2- ( ) .
. - . - , , .
«» «».
, «» , « » (, - ). .


, , Ariane- : Aérospatiale, Matra Marconi Space, Alenia, Spazio, DASA . «» .

, . «» , .
:

. : ( 40 ) .
( 0110, 0162. . - ) ().

«» ,
( , 2, ) . .
- .
- .
():
( ), - . :26- , , , YouTube. , , . .
() - :

: , .
:
.
, .. , ..

1. . : ..« . »
« , , » . .
, .
2. ( ) :

. . .
, () .
.. « ».
. .
. . sergiy_fakas

« , „“ (, ) .. . .
3. ..1 , .
Yes. „ “ „“. .
..
» "- ? () :

: , . () , .
.
: .
.. « », , «, 1989. , .
-
4. .
4.1. ( ) , .
,
4.2. „“ ( ):
! ( ), , .
…
61,016? ?
— ( , , CH3NHNH2) , . Those. .
2 — , - 2 Liquid oxygen, LOX
, .
5. „“ . , . , ., . ? ?
?
- 1500 .
- … . !
.
, .
«Geektimes» .
( ) . . . „ “ ( ). .
. . - .
Thanks to all.
, .. , ..

«», « », « » ..
1. . : ..« . »
« , , » . .
, .
2. ( ) :

. . .
, () .
.. « ».
. .
. . sergiy_fakas

« , „“ (, ) .. . .
3. ..1 , .
Yes. „ “ „“. .
..
» "- ? () :

: , . () , .
.
: .
.. « », , «, 1989. , .
-
4. .
4.1. ( ) , .
,
4.2. „“ ( ):
— 32
! ( ), , .
…
— 63,016
61,016? ?
, .
— ( , , CH3NHNH2) , . Those. .
— 2 ( LOX).
2 — , - 2 Liquid oxygen, LOX
, .
5. „“ . , . , ., . ? ?
?
- 1500 .
- … . !
: «», «», «» «»?
.
, .
«Geektimes» .
( ) . . . „ “ ( ). .
. . - .
Thanks to all.
:
technomag.bmstu.ru
www.abm-website-assets.s3.amazonaws.com
www.free-inform.ru
www.rusarchives.ru
www.epizodsspace.airbase.ru
www.polkovnik2000.narod.ru
www.avia-simply.ru
www.arms-expo.ru
www.npoenergomash.ru
www.buran.ru
www.fsmedia.imgix.net
www.wikimedia.org
www.youtu.be
www.cdn.tvc.ru
www.commi.narod.ru
www.dezinfo.net
www.nasa.gov
www.novosti-n.org
www.prirodasibiri.ru
www.radikal.ru
www.spacenews.com
www.esa.int
www.bse.sci-lib.com
www.kosmos-x.net.ru
www.rocketpolk44.narod.ru
www.criotehnika.ru
www..
www.chistoprudov.livejournal.com/104041.html
www.cryogenmash.ru
www.eldeprocess.ru
www.chemistry-chemists.com
www.rusvesna.su
www.arms-expo.ru
www.armedman.ru
www..
www.ec.europa.eu
www.mil.ru
www.kbkha.ru
www.naukarus.com
technomag.bmstu.ru
www.abm-website-assets.s3.amazonaws.com
www.free-inform.ru
www.rusarchives.ru
www.epizodsspace.airbase.ru
www.polkovnik2000.narod.ru
www.avia-simply.ru
www.arms-expo.ru
www.npoenergomash.ru
www.buran.ru
www.fsmedia.imgix.net
www.wikimedia.org
www.youtu.be
www.cdn.tvc.ru
www.commi.narod.ru
www.dezinfo.net
www.nasa.gov
www.novosti-n.org
www.prirodasibiri.ru
www.radikal.ru
www.spacenews.com
www.esa.int
www.bse.sci-lib.com
www.kosmos-x.net.ru
www.rocketpolk44.narod.ru
www.criotehnika.ru
www..
www.chistoprudov.livejournal.com/104041.html
www.cryogenmash.ru
www.eldeprocess.ru
www.chemistry-chemists.com
www.rusvesna.su
www.arms-expo.ru
www.armedman.ru
www..
www.ec.europa.eu
www.mil.ru
www.kbkha.ru
www.naukarus.com
Source: https://habr.com/ru/post/401795/
All Articles