Exotic states of matter, LCD displays and the future of water

Liquid crystals in the nematic phase
At school, you probably passed that a substance can be in three thermodynamic phases : solid, liquid and gaseous. (The term "phase" is used in conjunction with the term "state", and none of them has a clear, generally accepted definition). For young students, this is a useful simplification, but in fact there are much more phases. In the past hundred years we have discovered the existence of hundreds of different solid phases — some of them are used to create silicon chips in your computer. In addition, there are dozens of phases of liquid crystals - some of them create images on your screen. And we have not even touched truly exotic things: quantum phases, superfluid liquids, quark-gluon plasma, Bose-Einstein condensates and so-called. "Topological phases".
But first we will return to the beginning and discuss what the “phase” is. Like many fundamental concepts, it is better explained by example. Consider a glass of water in which some ice cubes float. There is only one substance in a glass: water. A lot of H 2 O molecules.
And although ice molecules are identical to the molecules in the surrounding water, there is obviously a big difference between them. The most obvious is that one of them is solid, keeping its shape, while the other freely flows and takes the form of a vessel. There is also a difference in density, electrical conductivity and a handful of other properties. Therefore, we say that liquid water and ice are different phases of the same substance. (Water is the only substance, with three different phases of which a person constantly collides. This is one of the reasons why water is a special substance).
')
How is it that ice and water show completely different behavior? You may be tempted to say that everything depends on the temperature: the water is warm, the ice is cold, and because of this, the water molecules behave differently. But at 0 ° C and normal atmospheric pressure, ice and liquid water are stable — that is, they can exist infinitely and not change.
But if the key difference is not the temperature, then what?
Large and small scales
Scientists have long argued about the relationship between the macroscopic properties of various materials, and the microscopic arrangement of the particles from which they are made. One of the earliest attempts was made in 1611 by Johann Kepler in his work with the beautiful name: “Hexagonal snowflake: the gift of the New Year”. Kepler argued that the six-fold symmetry of the snowflakes can be explained if we assume that ice is created from tiny spheres packed in hexagonal sets.
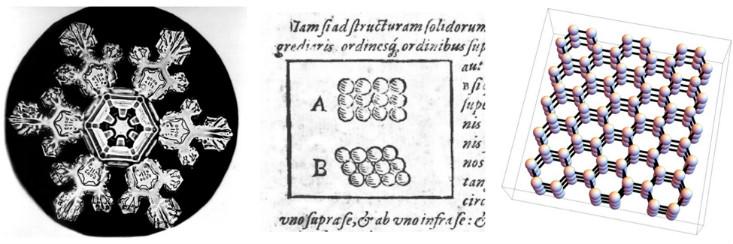
We now know that Kepler’s assumption about the particular arrangement of atoms was slightly incorrect — his scheme does not reflect the three-dimensional form and complex interaction of water molecules. (In his favor, we say that chemistry was not yet invented at that time, and the existence of atoms was finally proved almost 300 years later). However, he was on the right track. The sixfold symmetry of snowflakes comes from the fact that water molecules are lined up in a three-dimensional structure with sixfold symmetry (and there are also triangular snow crystals , and no one knows exactly why).
Speaking in the language of modern physics, Kepler suggested that snow is a crystalline solid substance, a phase of matter that receives its properties due to the fact that its atoms or molecules line up in periodic rows, or crystal lattices. Physicists use the word “crystal” to describe a microscopic structure, no matter whether the material looks like a diamond, a grain of sand, or a snowflake. Therefore, for physics, many metals and ceramics are crystals, since their atoms or molecules are lined up in a periodic lattice — in a certain three-dimensional version of Kepler’s 1611 drawings.
This explains the ice in a glass of water. What about liquid water? In any substance at a temperature above absolute zero (-273 ° C), the molecules constantly move and the word billiard balls bounce off each other after a strong breakdown. The temperature of a substance is a measure of the speed of movement of molecules.
In a liquid, this chaotic thermal motion is strong enough to overcome the forces that hold the molecules in the lattice. And without this lattice, molecules can move much more freely. This explains why the liquid gives in when you press it with your finger, but the ice does not. Interestingly, there are substances, in particular, window glass, with a completely disordered microstructure, but nevertheless acting at the macroscopic level, as solid. Understanding their behavior is the most important of the open questions of the physics of materials.
Fantastic phases
In three-dimensional materials - such as real ice, and not as on Kepler’s sketches - everything becomes more complicated, since in three dimensions there are many ways to arrange molecules into periodic structures. For example, there are about 17 ice phases (approximately, because this number depends on how to classify them), the newest of which was first created and studied by Italian scientists last year. Different phases arise at different temperatures and pressures, and although they are all solid, each of them has its own density, strength, reaction to electric and magnetic fields, thermal conductivity, etc. Because of the existence of these differences in macroscopic behavior, we call them different phases. Under "normal" conditions — temperature and pressure on the surface of the Earth — ice is usually in the form of ice I h . This stable hexagonal crystalline form is responsible for the sixfold symmetry of the snowflakes.
According to the marks of different phases of ice, ice IX actually exists. Fortunately, at temperature and pressure on the surface of the Earth, it is much more fragile than the other “ice nine” - a stable phase, due to which the oceans in Kurt Vonnegut's novel Cat's Cradle hardened dramatically.
And that's not all, even with such a familiar substance. Other, not yet open phases of ice can exist at ultrahigh pressures in the centers of gas giants like Uranus and Neptune. In such extreme conditions, at a pressure of 10 million atm. and more, water must form very strange crystalline solid phases. Among them there is a metallic conductive phase, which should look shiny if you figure out how to polish it.
Intermediate options
The relationship between microstructure and macro properties is the basic idea of science and engineering, it is used in the development of a variety of materials. For example, liquid crystals are a key component of laptops, televisions and smartphone screens. Liquid crystal molecules have an unusually long stick-like structure. Because of this, they can be in several different phases, with properties not found in ordinary materials.
For example, at high temperatures, the molecules are in disarray. Each "stick" is in a random place, like molecules in liquid water, and is oriented in an arbitrary direction. This is the isotropic phase. At lower temperatures, molecules can go into the nematic phase, in which they are randomly located, as in liquid water, but directed in one direction. Since no lattice is formed, the nematic phase flows like a liquid, but since the rods are directed in one direction, it has a microscopic order. Therefore, they are called "liquid crystals."
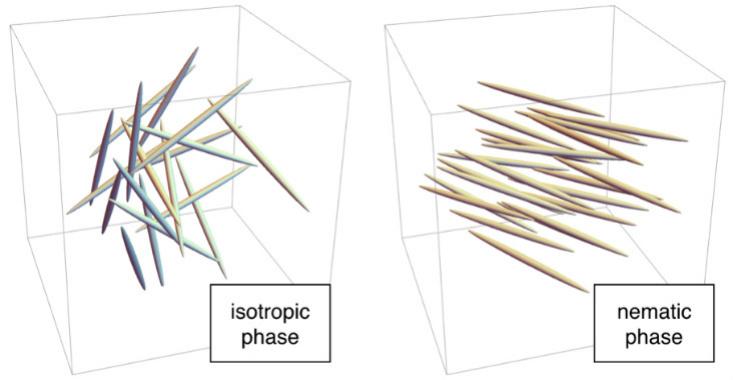
Fortunately for modern humans, the nematic phase has unusual properties associated with direction. When hit in an electric field, the molecules line up along it. In LCD screens, molecules in the nematic phase behave like a polarizing filter: a device that transmits light when the molecules are oriented in a particular way (for example, from top to bottom), and delays it when they are oriented perpendicularly. Since this work requires a polarizing filter, in phones and displays, a filter is placed between the light source and the screen. In LCD, tiny electrodes next to each pixel create an electric field that controls the position of the molecules inside it, and, therefore, the emission of light. This operation scheme is used in IPS-displays (“in-plane switching”). There are other options, for example, TN-displays, using even more exotic, twisted nematic phase.
Modeling miniature
It is clear that the microstructure helps to explain the properties of the materials that we meet and create. But can we see the microscopic order in real life? For ordinary substances, such as ice, salt or metal, it is difficult, because atoms and molecules are very small. A water molecule is less than one billionth of a meter - it cannot be seen with a conventional light microscope, and it is very difficult to see it even using modern microscopy techniques.
Fortunately, not only atoms and molecules can organize themselves into phases. In Chaikin's laboratory at the center of soft materials research at New York University, we use small hard spheres to study the phases of matter. They are called colloids, and they can be made from a variety of materials, such as glass, plastic, or metal. Colloids in the laboratory are 3,000 times larger than water molecules — they are comparable in size to bacteria or the nuclei of animal cells. For material experts, colloids are something like “ideal particles” - large enough to be visible through a microscope and small enough to form phases that are in many ways similar to conventional materials.
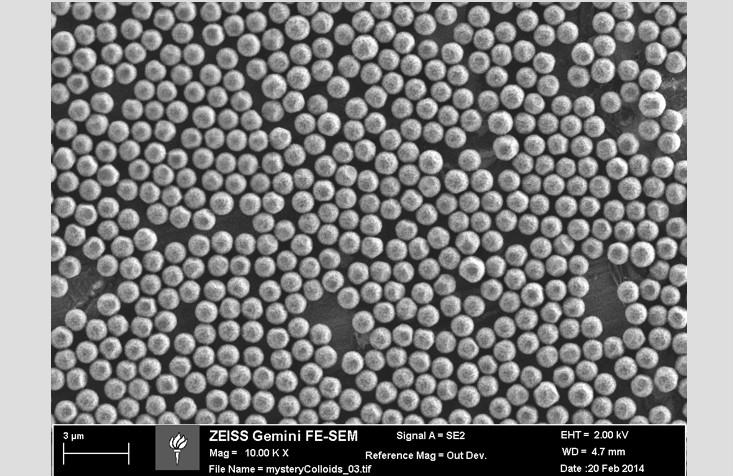
This photo shows plastic colloids coated with chemical composition, due to which they repel each other, being on the surface of the water
In our laboratory, we make colloids with a special coating that allows them to adhere to the water surface. When they stick to drops, we can make beautiful micrographs, such as this one. They can see the microscopic structure of various phases, and how their orientation leads to the appearance of such macroscopic properties as hardness. For example, we are now studying how the behavior of our particles changes depending on the use of spherical drops or flat surfaces of water. Does the formation of particles on the sphere help or hinder the formation of crystals and the appearance of systems like a solid body? This can help us understand the properties of important structures that are spherical or more complex in shape. If, for example, we knew more about the protein membranes that protect the HIV genes, we could learn to break them and defeat the virus.
When the crystals deteriorate
One of the most important stages in the study of a microscopic order is the study of the conditions under which order is disturbed. For example, if you look at the image of colloids in a crystal lattice, you can see that their order is not uniform. You can see the imperfections and irregularities, defects.

The image is made with a confocal microscope - a fluorescent dye is added to the sample, which is then illuminated with a laser
Similar defects occur in crystals of atoms or molecules, and they play an important role. For example, they determine the fragility of the material - how much load it can withstand before it breaks. In addition, since defects can affect the electrical conductivity of a material, semiconductor manufacturers spend billions of dollars on producing single-crystal silicon — giant silicon blocks with almost no defects. On the other hand, sometimes these microscopic flaws are very helpful. Recent studies have focused on the management and control of graphene defects to optimize it as a desalination filter.
We use colloidal crystals to observe how defects appear, move and interact with each other. As well as studying the phases, studying colloidal crystals can help us understand defects in other materials. As in many areas of life, sometimes imperfections are the most interesting part.
Source: https://habr.com/ru/post/401549/
All Articles