Build diagrams from biological switches
← Previous cycle article
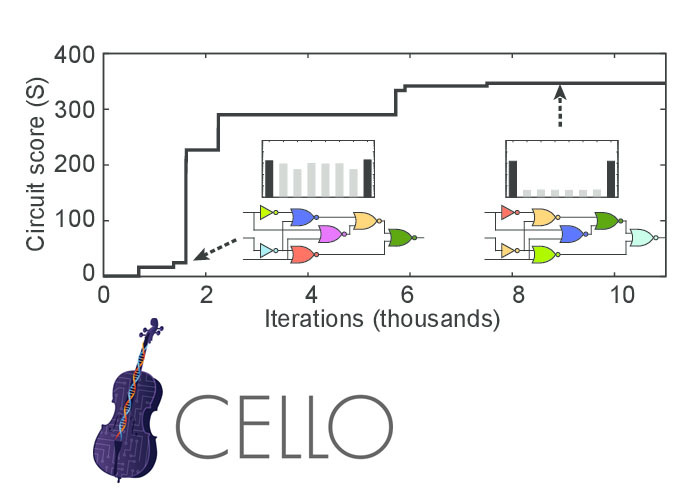
The logic of inclusion of operons of different metabolic pathways in Escherichia coli is close to the AND and OR elements. Therefore, based on them, you can make a gene construct that implements an arbitrary logic circuit (not too complicated). There are programs, such as Cello, which, from the description of logic in Verilog, make a sequence of gene constructs that implement this logic.
More difficult is the inverse problem: from the sequence of natural DNA to obtain a description of the logic of its regulation. Let's start with simple cases where there are no complex feedback loops and the integration of multiple signals on one operon. Almost all the regulation of E. coli genes satisfies these conditions. If we take all the known regulatory links between the genes of Escherichia coli and build a network of them, then we can calculate the statistics of the “motives” of this network (ways of connecting elements) and identify the most frequently used ones.
In the last article, we simulated the work of the lactose operon with a simple model with four parameters. Time was not part of this model. Therefore, it, in fact, describes only equilibrium states, when the levels of input signals remain constant for a long time. To simulate fast processes when turning on and off genes, additional factors must be taken into account.
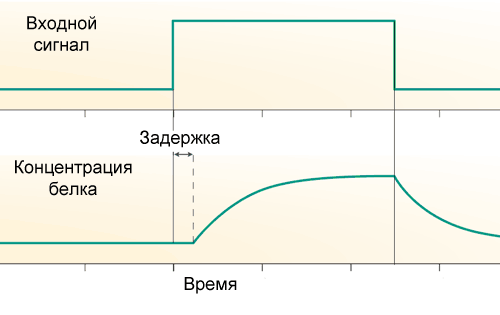
First, there is a transcriptional delay: from the start of RNA polymerase to the end of the synthesis of mRNA and then proteins, a considerable time elapses, about 10 minutes in Escherichia coli and from an hour or more in humans. Therefore, the rate of appearance of new protein molecules does not depend on the state of the promoter of its gene right now, but on the state of the promoter a few minutes ago.
')
Secondly, each protein is not only synthesized, but also breaks down. In the simplest case, the breakdown of the protein is not controlled and a fixed fraction of the molecules of this protein breaks down in the cell every minute. The more they eat, the more they fall apart. That is, the protein has a half-life, during which its concentration decreases by half.
To describe the dynamics of switching on and off the gene, we need to write diffur:
where A is the gene activity (that is the input function from the previous article), tau is the time of transcriptional delay, and k is the rate of protein breakdown. If A is first equal to zero, and then increases abruptly and keeps at a constant level, then changes in the amount of protein over time will look like this:
The simplest motif of E. coli, the feedback loop, consists of a single gene that regulates itself. More often the gene is a repressor for itself, that is, the feedback in the loop is negative. If this is the main regulatory input, then this feedback usually serves to stabilize the level of protein encoded by the gene. Such a feedback loop, for example, maintains a constant level of lactose repressor in the cell. If, in addition to negative feedback, there are other regulatory inputs, the feedback accelerates the reaction of the gene to an external signal.
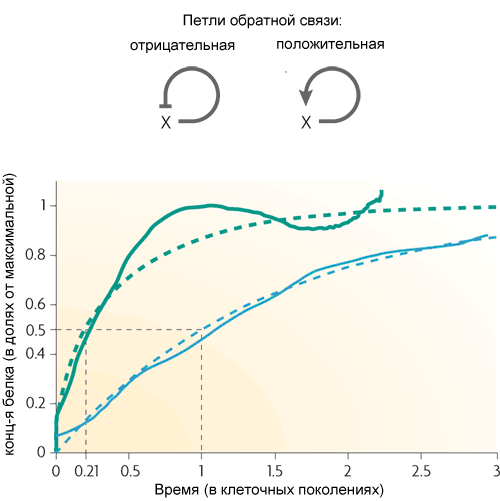
(the dotted lines are theoretical curves, the solid ones are the result of the experiment. Green ones - with negative feedback, blue ones - without it)
Positive feedback (protein activates its own gene) usually leads to triggering behavior. Such a gene has two stable states: it either does not work at all, or it works at full capacity. A strong but short external signal can transfer a gene from one steady state to another, in which it will remain until the next signal. Such a motive can serve as an element of memory. If a gene “catches” noise at a regulatory input, then a positive feedback will lead to a strong variation in gene activity between neighboring cells growing under the same conditions. This is used to maintain the heterogeneity of bacteria growing together so that sudden stress does not kill everyone at once.
For example, many antibiotics disrupt the process of protein synthesis. If a cell does not grow and does not synthesize proteins, then it can easily survive the addition of antibiotics to the environment and return to growth later. In E. coli, even in ideal conditions, up to 0.01% of the cells are hibernating in case of a sudden poisoning of the medium. These dormant cells (they are called "persistors") do not differ from the others in DNA, they enter and exit from hibernation under the control of a gene with positive feedback and input for noise.
Another common motif of gene networks is a bit more complicated. It is called the feed-forward loop and consists of three genes: the X gene regulates the Y and Z genes, the Y gene regulates the Z. Each of the three links in the loop can be activation or repression, therefore, in principle, there are 8 types such loops. Actually, there are usually two types of eight: either the X and Y genes are activators (coherent loop of type 1), or X is activator of Y and Z, Y is repressor of Z (non-coherent loop of type 1).
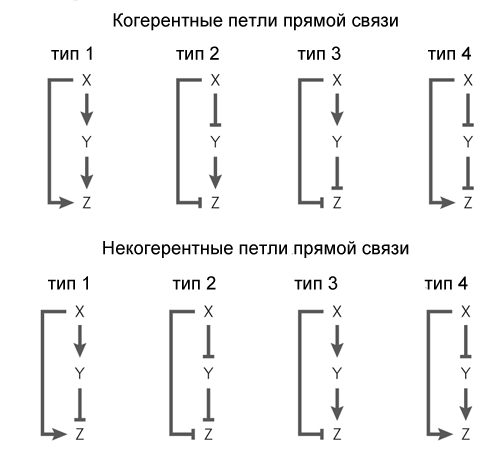
The behavior of the loop with two activators depends on the logic of the interaction of the inputs on Z. If the output gene in the loop works as AND, then the loop works as a low-pass filter that does not allow short pulses to pass to the output. In order for such a loop to produce an output signal, input (X) must receive a signal long enough for protein X to accumulate, and then protein Y. Only the combined action of proteins X and Y will turn on the Z gene.
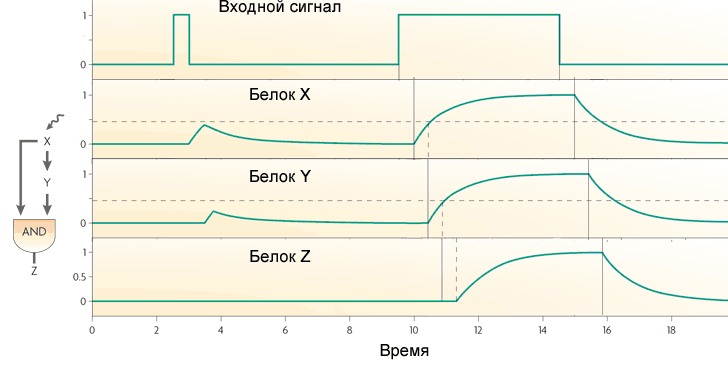
If the Z gene works as OR, then the loop function becomes opposite: it transmits all impulses to the output, and transforms short ones into long ones. But she does not miss short pauses between pulses.
The loop of direct communication with the activator X and the repressor Y works as a high-pass filter. It generates pulses of a small fixed length (equal to the transcriptional delay of the Y gene) in response to the leading edges of the input signals.
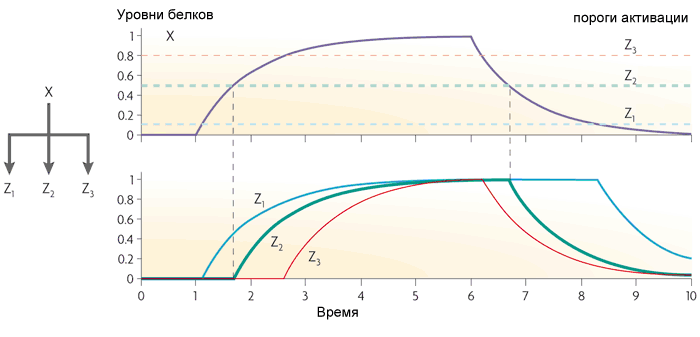
The third frequent motif in E. coli gene networks is a single-input module. It is simply a group of genes driven by the same regulatory gene. Typically, the genes in this group have different activation thresholds, so a smooth increase in the concentration of the transcription factor includes the genes in a certain order. Such a scheme is used, for example, in managing responses to stress.
The fourth, the most difficult motive is the multi-input module (multi-input module, or dense overlapping regulon). It consists of several regulatory genes that control several "output" genes.
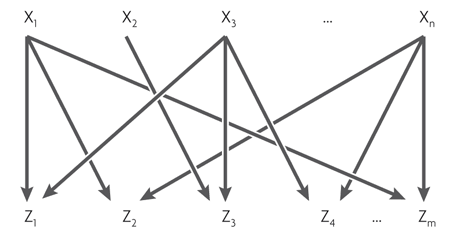
Depending on the logic of regulation of each output gene, this motif can work very differently. It can be compared with the assembly of logical elements, which, depending on the connections, can be a decoder, a counter, or something else. A complete description of the logic of the work of such motives does not yet exist.
These four motifs almost completely exhaust the structure of E. coli or yeast gene networks. Based on them, various artificial schemes are already being made. For example, bacteria with an oscillation generator, which is also synchronized in neighboring cells, and access to luminescent proteins is a complete analogue of “flashing a light bulb” in microcontrollers and “Hello World” in other programmers. Adding to this system a “sense of quorum” (when bacteria perceive how many fellows surround them), and changing the output from luminescence to toxins, the scientists collected bacteria that creep inside the cancer, and accumulate in large numbers, die together, releasing toxins and killing tumor ( more here ).
But it is bacteria and yeast. Animal gene networks are much more complex. In the next part, we will analyze one of the well-studied animal gene networks and the problems that arise with their study and modeling.
The logic of inclusion of operons of different metabolic pathways in Escherichia coli is close to the AND and OR elements. Therefore, based on them, you can make a gene construct that implements an arbitrary logic circuit (not too complicated). There are programs, such as Cello, which, from the description of logic in Verilog, make a sequence of gene constructs that implement this logic.

More difficult is the inverse problem: from the sequence of natural DNA to obtain a description of the logic of its regulation. Let's start with simple cases where there are no complex feedback loops and the integration of multiple signals on one operon. Almost all the regulation of E. coli genes satisfies these conditions. If we take all the known regulatory links between the genes of Escherichia coli and build a network of them, then we can calculate the statistics of the “motives” of this network (ways of connecting elements) and identify the most frequently used ones.
Gene switching dynamics
In the last article, we simulated the work of the lactose operon with a simple model with four parameters. Time was not part of this model. Therefore, it, in fact, describes only equilibrium states, when the levels of input signals remain constant for a long time. To simulate fast processes when turning on and off genes, additional factors must be taken into account.
First, there is a transcriptional delay: from the start of RNA polymerase to the end of the synthesis of mRNA and then proteins, a considerable time elapses, about 10 minutes in Escherichia coli and from an hour or more in humans. Therefore, the rate of appearance of new protein molecules does not depend on the state of the promoter of its gene right now, but on the state of the promoter a few minutes ago.
')
Secondly, each protein is not only synthesized, but also breaks down. In the simplest case, the breakdown of the protein is not controlled and a fixed fraction of the molecules of this protein breaks down in the cell every minute. The more they eat, the more they fall apart. That is, the protein has a half-life, during which its concentration decreases by half.
More details for especially curious
In growing cells, there is a second factor in the fall in protein concentration - dilution due to an increase in cell volume. It is described by the same formula as decay - the concentration decreases by a factor of two from one cell division to the next.
To describe the dynamics of switching on and off the gene, we need to write diffur:
where A is the gene activity (that is the input function from the previous article), tau is the time of transcriptional delay, and k is the rate of protein breakdown. If A is first equal to zero, and then increases abruptly and keeps at a constant level, then changes in the amount of protein over time will look like this:

Gene motifs and their functions
The simplest motif of E. coli, the feedback loop, consists of a single gene that regulates itself. More often the gene is a repressor for itself, that is, the feedback in the loop is negative. If this is the main regulatory input, then this feedback usually serves to stabilize the level of protein encoded by the gene. Such a feedback loop, for example, maintains a constant level of lactose repressor in the cell. If, in addition to negative feedback, there are other regulatory inputs, the feedback accelerates the reaction of the gene to an external signal.

(the dotted lines are theoretical curves, the solid ones are the result of the experiment. Green ones - with negative feedback, blue ones - without it)
Positive feedback (protein activates its own gene) usually leads to triggering behavior. Such a gene has two stable states: it either does not work at all, or it works at full capacity. A strong but short external signal can transfer a gene from one steady state to another, in which it will remain until the next signal. Such a motive can serve as an element of memory. If a gene “catches” noise at a regulatory input, then a positive feedback will lead to a strong variation in gene activity between neighboring cells growing under the same conditions. This is used to maintain the heterogeneity of bacteria growing together so that sudden stress does not kill everyone at once.
For example, many antibiotics disrupt the process of protein synthesis. If a cell does not grow and does not synthesize proteins, then it can easily survive the addition of antibiotics to the environment and return to growth later. In E. coli, even in ideal conditions, up to 0.01% of the cells are hibernating in case of a sudden poisoning of the medium. These dormant cells (they are called "persistors") do not differ from the others in DNA, they enter and exit from hibernation under the control of a gene with positive feedback and input for noise.
Another common motif of gene networks is a bit more complicated. It is called the feed-forward loop and consists of three genes: the X gene regulates the Y and Z genes, the Y gene regulates the Z. Each of the three links in the loop can be activation or repression, therefore, in principle, there are 8 types such loops. Actually, there are usually two types of eight: either the X and Y genes are activators (coherent loop of type 1), or X is activator of Y and Z, Y is repressor of Z (non-coherent loop of type 1).

The behavior of the loop with two activators depends on the logic of the interaction of the inputs on Z. If the output gene in the loop works as AND, then the loop works as a low-pass filter that does not allow short pulses to pass to the output. In order for such a loop to produce an output signal, input (X) must receive a signal long enough for protein X to accumulate, and then protein Y. Only the combined action of proteins X and Y will turn on the Z gene.

If the Z gene works as OR, then the loop function becomes opposite: it transmits all impulses to the output, and transforms short ones into long ones. But she does not miss short pauses between pulses.
The loop of direct communication with the activator X and the repressor Y works as a high-pass filter. It generates pulses of a small fixed length (equal to the transcriptional delay of the Y gene) in response to the leading edges of the input signals.
The third frequent motif in E. coli gene networks is a single-input module. It is simply a group of genes driven by the same regulatory gene. Typically, the genes in this group have different activation thresholds, so a smooth increase in the concentration of the transcription factor includes the genes in a certain order. Such a scheme is used, for example, in managing responses to stress.

The fourth, the most difficult motive is the multi-input module (multi-input module, or dense overlapping regulon). It consists of several regulatory genes that control several "output" genes.

Depending on the logic of regulation of each output gene, this motif can work very differently. It can be compared with the assembly of logical elements, which, depending on the connections, can be a decoder, a counter, or something else. A complete description of the logic of the work of such motives does not yet exist.
These four motifs almost completely exhaust the structure of E. coli or yeast gene networks. Based on them, various artificial schemes are already being made. For example, bacteria with an oscillation generator, which is also synchronized in neighboring cells, and access to luminescent proteins is a complete analogue of “flashing a light bulb” in microcontrollers and “Hello World” in other programmers. Adding to this system a “sense of quorum” (when bacteria perceive how many fellows surround them), and changing the output from luminescence to toxins, the scientists collected bacteria that creep inside the cancer, and accumulate in large numbers, die together, releasing toxins and killing tumor ( more here ).
But it is bacteria and yeast. Animal gene networks are much more complex. In the next part, we will analyze one of the well-studied animal gene networks and the problems that arise with their study and modeling.
Source: https://habr.com/ru/post/400899/
All Articles