Mathematics of Biological Switches
(continuation. Previous part: “Automation from DNA and proteins: what does it have in common with electronics?” )
We stopped at the fact that the lactose operon is similar to the logical element AND. But where do digital properties come from? After all, both input signals (concentrations of cAMP and lactose) are, in fact, analog. Let's try to draw the input functions of the lactose operon.
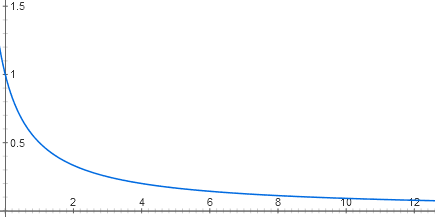
Each entry of the lactose repressor consists of two stages of molecular recognition. Lactose binds to the lactose repressor, and the repressor binds to DNA. There are several dozen repressor molecules in the cell, each of which, as long as there is no lactose, can be associated with the onset of the lactose operon. When it is connected there, RNA polymerase cannot start working. Due to the thermal movement of the repressor molecules, they constantly fall off from the DNA and join back. If there is no repressor at all, the operon works at full capacity (now, for simplicity, we believe that there is an excess of catabolic activator). At low concentrations of the repressor, it reduces the activity of the operon almost linearly. But then the effect of each new portion of the repressor is less and less, and in general, the dependence graph is close to the hyperbola y = 1 / (x + 1)
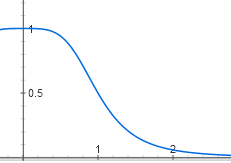
The repressor molecules float in the cell and bind to DNA only in bundles of four. In order for the active form of the repressor to become inactive, it must connect with four lactose molecules - one or two is not enough, one lactose molecule is needed for each protein subunit of the quadruplex repressor. While there is little lactose in the cell, its molecules bind to the repressor one to two and it remains active. But after some threshold concentration of lactose, the majority of repressor molecules bind four molecules of lactose each and become inactive. The graph of repressor activity depending on the amount of lactose is therefore S-shaped and is described by a function of the form y = 1 / (1 + x ^ 4).
Now, to get the input characteristic of the lactose operon, it is necessary to substitute the second function into the first one. It will be up to coefficients to have the following form:
y = 1 / (1 + (1 / (1 + x ^ 4)))
Her schedule is also S-shaped. It turns out that the lactose operon does not respond to small concentrations of lactose. When the threshold concentration is reached, inactive repressor molecules appear, linking four lactose each, and the operon switches on quite sharply.
The second regulatory input of the lactose operon also consists of two steps of molecular recognition: cAMP is associated with an activator, and the activator is associated with DNA. The difference from lactose entry is that here the binding of molecules (activator or cAMP) increases the activity of what they are associated with (operon or activator). That is, the graphs will go out of zero and approach the horizontal line somewhere above the X axis.
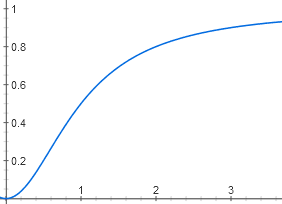
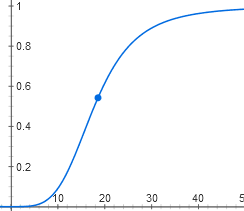
Due to the binding of two cAMP molecules, the graph does not look like a hyperbole, but as an S-shaped curve. Although the threshold effect on it is less noticeable than on the graph for lactose, because the degree is not the fourth, but only the second.
The dependence of the activity of the operon on the amount of activator has a different nuance. If the repressor binding suppresses the operon almost completely (somewhere up to 0.1%, which is lower than the biochemical measurement error), then the absence of the activator suppresses activity only up to 5%. Therefore, the graph will not go out of zero, but from a point (0; 0.05):
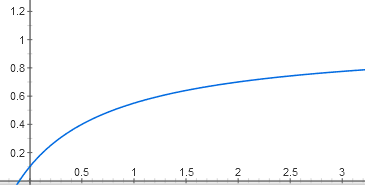
y = 0.05 + (0.95 * x / (1 + x))
It remains to obtain the dependence of the activity of the operon on the concentration of cAMP. To do this, substitute the first formula (for the concentration of active CAP depending on the concentration of cAMP) in the second formula (for the activity of the operon on the concentration of active SAR) ...
... And get a four-story fraction:
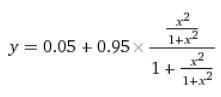
We have the most difficult function of all in this article. However, its graph looks similar to the graph of a simpler dependence of the active CAP on the concentration of cAMP, y = x ^ 2 / (1 + x ^ 2):
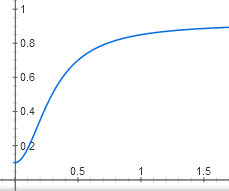
It is also an S-shaped curve with a certain threshold effect, which slowly approaches the horizontal line y = 1. It only begins not from zero, but from a point (0; 0.05).
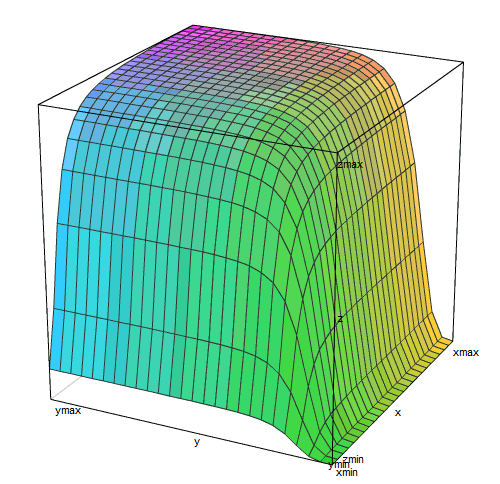
It remains to understand how the two inputs interact. In the case of lactose operon, the answer is simple - no way. Lactose repressor and catabolic activator do not affect the binding of each other to DNA. Therefore, the binding of these two proteins can be considered independent events. The full activity of the operon is achieved when both the activator is bound and the repressor is not bound. The probability of such a coincidence is equal to the product of the probabilities of each of them separately. So, to get the activity function of the lactose operon from two variables ([Lac]) and ([cAMP]), you just have to multiply the functions from each of these variables:
Activity = (1 / (1 + (1 / (1 + [Lac] ^ 4)))) * (0.05 + 0.095 * ([cAMP] ^ 2 / (1 + [cAMP] ^ 2)) / ( 1 + ([cAMP] ^ 2 / (1 + [cAMP] ^ 2))))
3D graph of this feature

similar to the plateau, terminated by gorges on two sides. The right ravine (small concentrations of lactose) is deeper and flat-bottomed than the left (small concentrations of cAMP).
In real life, E. coli around it lactose is either not at all (most often), or its concentration is higher than the threshold and the lactose repressor practically does not interfere with the work of the operon (when the owner ate something dairy). The concentration of cAMP is an internal signal that is produced by the cell itself. It is also either too small to include a lactose operon (when there is glucose or starch), or sufficient to include 95% or more (if there is nothing tastier than lactose). That is, almost always the lactose operon is in the conditions of either a plateau on this graph, or in one of the gorges.
The input functions of the lactose operon are measured experimentally. The measurement method resembles the debugging of microcontrollers "hang LED on the output leg." The regulatory region of the operon is taken and fused to the green fluorescent protein gene. This gene construct is inserted into the cells of Escherichia coli, from which the regular lactose operon is removed (so that the concentration of lactose is fixed by the experimenter) and the standard system that encodes hunger levels of cAMP. After this, the activity of the lactose operon can be accurately measured by a spectrophotometer by the level of green fluorescence. It turns out that within the limits of measurement accuracy (1%), the theory is completely consistent with experiment ).
The bottom of the left gorge in the graph of the input function (at low concentrations of cAMP) is above zero. This is not a bug, but a feature: thanks to it, in the presence of several sugars at once, E. coli turns out to be ready to quickly switch to lactose when glucose runs out. E. coli mutants, which have a regulatory function of the lactose operon closer to pure AND (that is, without the cAMP, the operon does not work), switch from glucose to lactose for an hour or more: at the time of glucose exhaustion, they do not have lactose digestion enzymes, which means that there is no energy to make them quickly. Normal E. coli ("wild type") meets this moment, already having some enzymes of lactose absorption, and it takes 15-20 minutes to completely switch to a new sugar.
Now let's digress from the lactose operon and see more broadly what the logical elements on such a chemical base are capable of. Almost everywhere in biochemical signaling systems, we encounter molecular recognition, which is described by functions of the form y = x ^ n / (1 + x ^ n) for activators and y = 1 / (1 + x ^ n) for repressors. The degree n in these functions indicates the number of molecules of the same type to be bonded to produce an effect, and is usually 1, 2, or 4 (most often 2). You can get more complex logical keys by adding more binding sites for regulatory proteins to the beginning of the operon. These proteins may be more than two types. The effects of different proteins on the landing of RNA polymerase can fold (OR) or multiply (AND). But in general, we will deal with some combination (addition or multiplication) of the basic functions y = x ^ n / (1 + x ^ n) and y = 1 / (1 + x ^ n) with different powers and coefficients.
Some interesting features are very simple. For example, the same regulatory protein may bind (in paired form) with two promoter sites. In one area it acts as a repressor, and in the other as an activator. For the operon to work, it is necessary that the repressor site is empty, and the activator site is busy. It turns out the function with a clear maximum:
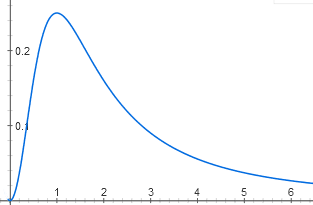
y = (1 / (1 + x ^ 2)) * (x ^ 2 / (1 + x ^ 2))
Without this protein, the operon is inactive, because the activator site is empty, and at high concentrations it is inactive, because the repressor site is occupied. The maximum activity will be when both the activator site and the repressor site are occupied with a 50% probability.
You can also make two activator binding sites, in one activator it will strongly bind to DNA, but weakly activate the gene, in the other - weakly attach to DNA, but strongly activate the gene. In this case, you get a function like y = x ^ 4 / (1 + x ^ 4) + 0.3 * (10x) ^ 4 / (1 + (10x) ^ 4), with a stepped graph:
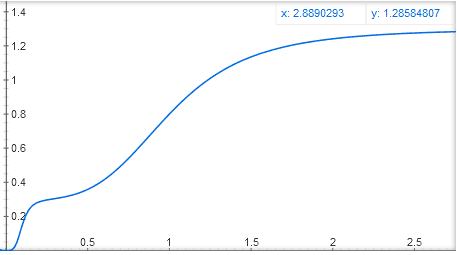
Mathematicians, ay! Can you tell in the comments what else is possible and what cannot be obtained by adding and multiplying these basic functions y = x ^ n / (1 + x ^ n) and y = 1 / (1 + x ^ n)?
In the next part we will deal with schemes of such logical elements.

We stopped at the fact that the lactose operon is similar to the logical element AND. But where do digital properties come from? After all, both input signals (concentrations of cAMP and lactose) are, in fact, analog. Let's try to draw the input functions of the lactose operon.
Each entry of the lactose repressor consists of two stages of molecular recognition. Lactose binds to the lactose repressor, and the repressor binds to DNA. There are several dozen repressor molecules in the cell, each of which, as long as there is no lactose, can be associated with the onset of the lactose operon. When it is connected there, RNA polymerase cannot start working. Due to the thermal movement of the repressor molecules, they constantly fall off from the DNA and join back. If there is no repressor at all, the operon works at full capacity (now, for simplicity, we believe that there is an excess of catabolic activator). At low concentrations of the repressor, it reduces the activity of the operon almost linearly. But then the effect of each new portion of the repressor is less and less, and in general, the dependence graph is close to the hyperbola y = 1 / (x + 1)

matan
Binding of the repressor to DNA can be considered a reversible chemical reaction. The equilibrium position in reversible chemical reactions is described by the law of mass action. What it is?
')
For example, we have a reaction in which substance C and substance C are formed. The reaction is reversible, that is, C can decompose back to A and B under the same conditions:
A + B <-> C
In the flask where this reaction takes place, some kind of balance will be established between the direct and reverse direction of the reaction. In the system there will be all three substances A, B and C in some concentrations (in physical chemistry, the concentration of a substance is usually denoted by its symbol in square brackets, [A], [B] and [C] in our example). The law of active masses connects the equilibrium concentrations of substances with each other: the product of the product concentrations divided by the product of the concentration of the initial substances is constant.
[C] / ([A] * [B]) = K
The letter K here indicates the reaction equilibrium constant. It depends only on the chemical nature of the reaction (i.e. which substances react) and on temperature. Concentrations of substances, pressure, catalysts and other factors do not affect the equilibrium constant.
For example, if we add more initial substance A to the flask, then the equilibrium will shift towards substance C, its concentration will increase, and the concentration of substance B (which we have not added) will fall. However, the ratio of [C] to ([A] * [B]) remains constant.
So, we have a reversible reaction of binding the active form of the repressor to the operon: RepA + OpA <-> Op (binding the repressor turns the active form of the operon into inactive). It has its own equilibrium constant K {RO} = [Op] / ([OpA] * [RepA]). We need to obtain from this the dependence of [OA] (the amount of the active form of the operon) on [RepA] (concentration of the repressor). We also know that there is only one lactose operon in a cell, which can be in active or inactive form: [Op] + [OpA] = 1
From the second equation we express [Op] through [OpA] and substitute it into the first equation:
K {RO} = (1 - [OpA]) / ([OpA] * [RepA])
After transformations we get:
[OpA] = 1 / (K {RO} * [RepA] + 1)
That is, the function has the form y = 1 / (x + 1)
')
For example, we have a reaction in which substance C and substance C are formed. The reaction is reversible, that is, C can decompose back to A and B under the same conditions:
A + B <-> C
In the flask where this reaction takes place, some kind of balance will be established between the direct and reverse direction of the reaction. In the system there will be all three substances A, B and C in some concentrations (in physical chemistry, the concentration of a substance is usually denoted by its symbol in square brackets, [A], [B] and [C] in our example). The law of active masses connects the equilibrium concentrations of substances with each other: the product of the product concentrations divided by the product of the concentration of the initial substances is constant.
[C] / ([A] * [B]) = K
The letter K here indicates the reaction equilibrium constant. It depends only on the chemical nature of the reaction (i.e. which substances react) and on temperature. Concentrations of substances, pressure, catalysts and other factors do not affect the equilibrium constant.
For example, if we add more initial substance A to the flask, then the equilibrium will shift towards substance C, its concentration will increase, and the concentration of substance B (which we have not added) will fall. However, the ratio of [C] to ([A] * [B]) remains constant.
So, we have a reversible reaction of binding the active form of the repressor to the operon: RepA + OpA <-> Op (binding the repressor turns the active form of the operon into inactive). It has its own equilibrium constant K {RO} = [Op] / ([OpA] * [RepA]). We need to obtain from this the dependence of [OA] (the amount of the active form of the operon) on [RepA] (concentration of the repressor). We also know that there is only one lactose operon in a cell, which can be in active or inactive form: [Op] + [OpA] = 1
From the second equation we express [Op] through [OpA] and substitute it into the first equation:
K {RO} = (1 - [OpA]) / ([OpA] * [RepA])
After transformations we get:
[OpA] = 1 / (K {RO} * [RepA] + 1)
That is, the function has the form y = 1 / (x + 1)
The repressor molecules float in the cell and bind to DNA only in bundles of four. In order for the active form of the repressor to become inactive, it must connect with four lactose molecules - one or two is not enough, one lactose molecule is needed for each protein subunit of the quadruplex repressor. While there is little lactose in the cell, its molecules bind to the repressor one to two and it remains active. But after some threshold concentration of lactose, the majority of repressor molecules bind four molecules of lactose each and become inactive. The graph of repressor activity depending on the amount of lactose is therefore S-shaped and is described by a function of the form y = 1 / (1 + x ^ 4).

Matan
Now we find the dependence of [RepA] on the concentration of lactose [Lac]. It is also derived from the law of the masses, with one subtlety: four identical lactose molecules are involved in binding to the repressor.
RepA + 4 Lac <-> Rep
In the equation for the equilibrium constant in this case, the following degrees appear:
K {RL} = [Rep] / ([RepA] * [Lac] ^ 4)
Again we use the fact that the total concentration of both forms of the repressor is constant:
[Rep] + [RepA] = n, where n ≈ 50
And we get that [RepA] = n / (K {RL} * [Lac] ^ 4 + 1)
RepA + 4 Lac <-> Rep
In the equation for the equilibrium constant in this case, the following degrees appear:
K {RL} = [Rep] / ([RepA] * [Lac] ^ 4)
Again we use the fact that the total concentration of both forms of the repressor is constant:
[Rep] + [RepA] = n, where n ≈ 50
And we get that [RepA] = n / (K {RL} * [Lac] ^ 4 + 1)
Now, to get the input characteristic of the lactose operon, it is necessary to substitute the second function into the first one. It will be up to coefficients to have the following form:
y = 1 / (1 + (1 / (1 + x ^ 4)))

Her schedule is also S-shaped. It turns out that the lactose operon does not respond to small concentrations of lactose. When the threshold concentration is reached, inactive repressor molecules appear, linking four lactose each, and the operon switches on quite sharply.
The second regulatory input of the lactose operon also consists of two steps of molecular recognition: cAMP is associated with an activator, and the activator is associated with DNA. The difference from lactose entry is that here the binding of molecules (activator or cAMP) increases the activity of what they are associated with (operon or activator). That is, the graphs will go out of zero and approach the horizontal line somewhere above the X axis.
Matan
Since the cAMP binding involves an activator, the active form will be the product of the reaction and not the starting material:
2 camp + Akt <-> AktA
The equilibrium constant K {Ts-A} = [AktA] / ([Akt] * [cAMP] ^ 2)
Again, we use the fact that the sum of the concentrations of [AktA] and [Akt] is constant, since there are about 100 activator molecules in the cell, passing between the active and inactive forms. Expressing [Akt] through [AktA], we get:
K {C-A} = [AktA] / ((100 - [AktA]) * [cAMP] ^ 2)
[AktA] = K {C-A} * [cAMP] ^ 2 / (1 + K {C-A} * [cAMP] ^ 2)
That is, the dependence of [AktA] on [cAMP] is of the form y = x ^ 2 / (1 + x ^ 2)
2 camp + Akt <-> AktA
The equilibrium constant K {Ts-A} = [AktA] / ([Akt] * [cAMP] ^ 2)
Again, we use the fact that the sum of the concentrations of [AktA] and [Akt] is constant, since there are about 100 activator molecules in the cell, passing between the active and inactive forms. Expressing [Akt] through [AktA], we get:
K {C-A} = [AktA] / ((100 - [AktA]) * [cAMP] ^ 2)
[AktA] = K {C-A} * [cAMP] ^ 2 / (1 + K {C-A} * [cAMP] ^ 2)
That is, the dependence of [AktA] on [cAMP] is of the form y = x ^ 2 / (1 + x ^ 2)

Due to the binding of two cAMP molecules, the graph does not look like a hyperbole, but as an S-shaped curve. Although the threshold effect on it is less noticeable than on the graph for lactose, because the degree is not the fourth, but only the second.
The dependence of the activity of the operon on the amount of activator has a different nuance. If the repressor binding suppresses the operon almost completely (somewhere up to 0.1%, which is lower than the biochemical measurement error), then the absence of the activator suppresses activity only up to 5%. Therefore, the graph will not go out of zero, but from a point (0; 0.05):

y = 0.05 + (0.95 * x / (1 + x))
Matan
The state of the operon “no activator, no repressor” also contributes to the synthesis of mRNA and the corresponding proteins, therefore
Activity = [OpA] + 0.05 * [Op]
The dependence of [OpA] on [AktA] we can easily derive by analogy with the previous three. The activator is connected one by one, so [AktA] will be included in the formula in the first degree. It activates the operon, so [AktA] will be both in the numerator and denominator:
[OpA] = K {A-O} * [AktA] / (K {A-O} * [AktA] + 1)
That is, the function has the form y = x / (1 + x)
And this is only the contribution of the operon associated with the activator! To take into account the contribution of the operon without an activator, one must add the addendum (1 is this fraction) * 0.05. Fortunately, both terms have a common denominator, so the function is complicated quite a bit:
y = 0.05 + (0.95 * x / (1 + x))
Activity = [OpA] + 0.05 * [Op]
The dependence of [OpA] on [AktA] we can easily derive by analogy with the previous three. The activator is connected one by one, so [AktA] will be included in the formula in the first degree. It activates the operon, so [AktA] will be both in the numerator and denominator:
[OpA] = K {A-O} * [AktA] / (K {A-O} * [AktA] + 1)
That is, the function has the form y = x / (1 + x)
And this is only the contribution of the operon associated with the activator! To take into account the contribution of the operon without an activator, one must add the addendum (1 is this fraction) * 0.05. Fortunately, both terms have a common denominator, so the function is complicated quite a bit:
y = 0.05 + (0.95 * x / (1 + x))
It remains to obtain the dependence of the activity of the operon on the concentration of cAMP. To do this, substitute the first formula (for the concentration of active CAP depending on the concentration of cAMP) in the second formula (for the activity of the operon on the concentration of active SAR) ...
... And get a four-story fraction:
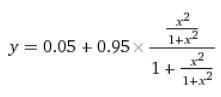
We have the most difficult function of all in this article. However, its graph looks similar to the graph of a simpler dependence of the active CAP on the concentration of cAMP, y = x ^ 2 / (1 + x ^ 2):

It is also an S-shaped curve with a certain threshold effect, which slowly approaches the horizontal line y = 1. It only begins not from zero, but from a point (0; 0.05).
It remains to understand how the two inputs interact. In the case of lactose operon, the answer is simple - no way. Lactose repressor and catabolic activator do not affect the binding of each other to DNA. Therefore, the binding of these two proteins can be considered independent events. The full activity of the operon is achieved when both the activator is bound and the repressor is not bound. The probability of such a coincidence is equal to the product of the probabilities of each of them separately. So, to get the activity function of the lactose operon from two variables ([Lac]) and ([cAMP]), you just have to multiply the functions from each of these variables:
Activity = (1 / (1 + (1 / (1 + [Lac] ^ 4)))) * (0.05 + 0.095 * ([cAMP] ^ 2 / (1 + [cAMP] ^ 2)) / ( 1 + ([cAMP] ^ 2 / (1 + [cAMP] ^ 2))))
3D graph of this feature

similar to the plateau, terminated by gorges on two sides. The right ravine (small concentrations of lactose) is deeper and flat-bottomed than the left (small concentrations of cAMP).
In real life, E. coli around it lactose is either not at all (most often), or its concentration is higher than the threshold and the lactose repressor practically does not interfere with the work of the operon (when the owner ate something dairy). The concentration of cAMP is an internal signal that is produced by the cell itself. It is also either too small to include a lactose operon (when there is glucose or starch), or sufficient to include 95% or more (if there is nothing tastier than lactose). That is, almost always the lactose operon is in the conditions of either a plateau on this graph, or in one of the gorges.
The input functions of the lactose operon are measured experimentally. The measurement method resembles the debugging of microcontrollers "hang LED on the output leg." The regulatory region of the operon is taken and fused to the green fluorescent protein gene. This gene construct is inserted into the cells of Escherichia coli, from which the regular lactose operon is removed (so that the concentration of lactose is fixed by the experimenter) and the standard system that encodes hunger levels of cAMP. After this, the activity of the lactose operon can be accurately measured by a spectrophotometer by the level of green fluorescence. It turns out that within the limits of measurement accuracy (1%), the theory is completely consistent with experiment ).
The bottom of the left gorge in the graph of the input function (at low concentrations of cAMP) is above zero. This is not a bug, but a feature: thanks to it, in the presence of several sugars at once, E. coli turns out to be ready to quickly switch to lactose when glucose runs out. E. coli mutants, which have a regulatory function of the lactose operon closer to pure AND (that is, without the cAMP, the operon does not work), switch from glucose to lactose for an hour or more: at the time of glucose exhaustion, they do not have lactose digestion enzymes, which means that there is no energy to make them quickly. Normal E. coli ("wild type") meets this moment, already having some enzymes of lactose absorption, and it takes 15-20 minutes to completely switch to a new sugar.
Now let's digress from the lactose operon and see more broadly what the logical elements on such a chemical base are capable of. Almost everywhere in biochemical signaling systems, we encounter molecular recognition, which is described by functions of the form y = x ^ n / (1 + x ^ n) for activators and y = 1 / (1 + x ^ n) for repressors. The degree n in these functions indicates the number of molecules of the same type to be bonded to produce an effect, and is usually 1, 2, or 4 (most often 2). You can get more complex logical keys by adding more binding sites for regulatory proteins to the beginning of the operon. These proteins may be more than two types. The effects of different proteins on the landing of RNA polymerase can fold (OR) or multiply (AND). But in general, we will deal with some combination (addition or multiplication) of the basic functions y = x ^ n / (1 + x ^ n) and y = 1 / (1 + x ^ n) with different powers and coefficients.
Some interesting features are very simple. For example, the same regulatory protein may bind (in paired form) with two promoter sites. In one area it acts as a repressor, and in the other as an activator. For the operon to work, it is necessary that the repressor site is empty, and the activator site is busy. It turns out the function with a clear maximum:

y = (1 / (1 + x ^ 2)) * (x ^ 2 / (1 + x ^ 2))
Without this protein, the operon is inactive, because the activator site is empty, and at high concentrations it is inactive, because the repressor site is occupied. The maximum activity will be when both the activator site and the repressor site are occupied with a 50% probability.
You can also make two activator binding sites, in one activator it will strongly bind to DNA, but weakly activate the gene, in the other - weakly attach to DNA, but strongly activate the gene. In this case, you get a function like y = x ^ 4 / (1 + x ^ 4) + 0.3 * (10x) ^ 4 / (1 + (10x) ^ 4), with a stepped graph:

Mathematicians, ay! Can you tell in the comments what else is possible and what cannot be obtained by adding and multiplying these basic functions y = x ^ n / (1 + x ^ n) and y = 1 / (1 + x ^ n)?
In the next part we will deal with schemes of such logical elements.
Source: https://habr.com/ru/post/399909/
All Articles