Lithium-sulfur batteries for future space programs
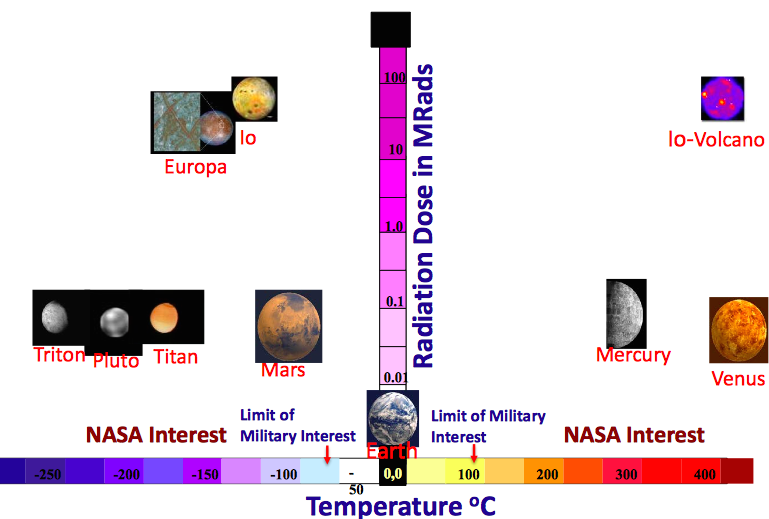
Today, batteries in space programs are mainly used as backup power sources, when the devices are in the shade and cannot receive energy from solar batteries, or in space suits for going into outer space. But the types of batteries used today (Li-ion, Ni-H 2 ) have a number of limitations. Firstly, they are too cumbersome, since preference is given not to energy intensity, but to safety, as a result, multiple protective mechanisms do not contribute to a decrease in volume at all. And secondly, modern batteries have temperature limitations, and in future programs, depending on the location, temperatures can vary in the range from -150 ° C to +450 ° C.
A source
In addition, do not forget the increased background radiation. In general, future batteries for the space industry should not only be compact, durable, safe and energy-intensive, but also work at high or low temperatures, as well as in conditions of high background radiation. Naturally, today such magic technology does not exist. But nevertheless, there are promising scientific developments that are trying to get closer to the requirements for future programs. In particular, I would like to talk about one direction in research, which is supported by NASA in the framework of the Game Changing Development (GCD) program.
Since it is difficult to combine all the above specifications in one battery-task, the main goal of NASA today is to get more compact, energy-intensive, and safe batteries. How to achieve this goal?
First of all, batteries with fundamentally new materials for electrodes are required for a significant increase in energy consumption per unit volume, since the capabilities of lithium-ion batteries (Li-ion) are limited by the capacities of materials for the cathode (about 250 mAh / g for oxides) and the anode ( about 370 mAh / g for graphite), as well as the voltage limits in which the electrolyte is stable. And one of the technologies, which allows to increase the capacity, using fundamentally new reactions instead of intercalation on the electrodes is lithium-sulfur batteries (Li-S), the anode of which contains metallic lithium, and sulfur is used as the active material for the cathode. The work of a lithium-sulfur battery is somewhat similar to the work of a lithium-ion battery: both there and there lithium ions are involved in charge transfer. But unlike Li-ion, ions in Li-S are not embedded in the layered structure of the cathode, but enter into the following reaction with it:
')
Although in practice, the reaction at the cathode rather looks like this:

A source
The main advantage of such a battery is a high capacity exceeding the capacity of lithium-ion batteries by 2-3 times. But in practice, not everything is so rosy. When recharging, lithium ions are deposited on the anode as horrible, forming metal chains (dendrites), which eventually lead to a short circuit. In addition, reactions between lithium and sulfur at the cathode lead to large changes in the volume of the material (up to 80%), so the electrode quickly collapses, and the compounds themselves with sulfur are poor conductors, therefore a lot of carbon material has to be added to the cathode. Finally, and most importantly, the intermediate reaction products (polysulfides) gradually dissolve in the organic electrolyte and “travel” between the anode and the cathode, which leads to a very strong self-discharge.
But all of the above problems are trying to solve a group of scientists from the University of Maryland (UMD), which won a grant from NASA. So how do scientists come to solving all these problems? First, they decided to “attack” one of the main problems of lithium-sulfur batteries, namely, self-discharge. And instead of a liquid organic electrolyte, which, as mentioned above, gradually dissolves the active materials, they used a solid ceramic electrolyte, or rather, Li 6 PS 5 Cl, which conducts lithium ions quite well through its crystal lattice.
But if solid electrolytes solve one problem, they also create additional difficulties. For example, large changes in the cathode volume during the reaction can lead to a rapid loss of contact between the solid electrode and the electrolyte, and a sharp drop in battery capacity. Therefore, scientists have proposed an elegant solution: they created a nanocomposite consisting of nanoparticles of cathode active material (LI 2 S) and electrolyte (Li 6 PS 5 Cl) enclosed in a carbon matrix.
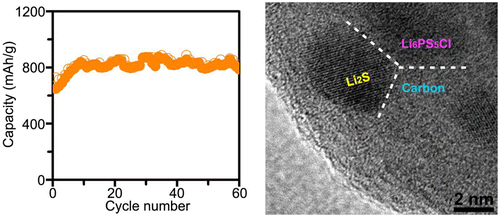
A source
This nanocomposite has the following advantages: firstly, the distribution of nanoparticles of the material, which changes in volume upon reaction with lithium, in carbon, the volume of which practically does not change, improves the mechanical properties of the nanocomposite (plasticity and strength) and reduces the risk of cracking. In addition, carbon not only improves conductivity, but also does not prevent the movement of lithium ions, since it also has good ionic conductivity. A due to the fact that the active materials are nanostructured, lithium does not need to move long distances to react, and the entire volume of material is used more efficiently. Finally, the use of such a composite improves the contact between the electrolyte, the active material, and the conductive carbon.
As a result, scientists received a completely solid battery with a capacity of about 830 mAh / g. Of course, it’s too early to talk about launching such a battery into space, since such a battery works for only 60 charge / discharge cycles. But at the same time, despite such a rapid loss of capacity, 60 cycles is already a significant improvement over previous results, since before this solid lithium-sulfur batteries did not work for more than 20 cycles. It should also be noted that such solid electrolytes can work in a large temperature range (by the way, they work best at temperatures above 100 ° C), so that the temperature limitations of such batteries will be due to active materials rather than electrolyte, which distinguishes such systems from batteries that use organic solutions in the form of an electrolyte.

A source
In addition, do not forget the increased background radiation. In general, future batteries for the space industry should not only be compact, durable, safe and energy-intensive, but also work at high or low temperatures, as well as in conditions of high background radiation. Naturally, today such magic technology does not exist. But nevertheless, there are promising scientific developments that are trying to get closer to the requirements for future programs. In particular, I would like to talk about one direction in research, which is supported by NASA in the framework of the Game Changing Development (GCD) program.
Since it is difficult to combine all the above specifications in one battery-task, the main goal of NASA today is to get more compact, energy-intensive, and safe batteries. How to achieve this goal?
First of all, batteries with fundamentally new materials for electrodes are required for a significant increase in energy consumption per unit volume, since the capabilities of lithium-ion batteries (Li-ion) are limited by the capacities of materials for the cathode (about 250 mAh / g for oxides) and the anode ( about 370 mAh / g for graphite), as well as the voltage limits in which the electrolyte is stable. And one of the technologies, which allows to increase the capacity, using fundamentally new reactions instead of intercalation on the electrodes is lithium-sulfur batteries (Li-S), the anode of which contains metallic lithium, and sulfur is used as the active material for the cathode. The work of a lithium-sulfur battery is somewhat similar to the work of a lithium-ion battery: both there and there lithium ions are involved in charge transfer. But unlike Li-ion, ions in Li-S are not embedded in the layered structure of the cathode, but enter into the following reaction with it:
')
2 Li + S -> Li 2 S
Although in practice, the reaction at the cathode rather looks like this:
S 8 -> Li 2 S 8 -> Li 2 S 6 -> Li 2 S 4 -> Li 2 S 2 -> Li 2 S

A source
The main advantage of such a battery is a high capacity exceeding the capacity of lithium-ion batteries by 2-3 times. But in practice, not everything is so rosy. When recharging, lithium ions are deposited on the anode as horrible, forming metal chains (dendrites), which eventually lead to a short circuit. In addition, reactions between lithium and sulfur at the cathode lead to large changes in the volume of the material (up to 80%), so the electrode quickly collapses, and the compounds themselves with sulfur are poor conductors, therefore a lot of carbon material has to be added to the cathode. Finally, and most importantly, the intermediate reaction products (polysulfides) gradually dissolve in the organic electrolyte and “travel” between the anode and the cathode, which leads to a very strong self-discharge.
But all of the above problems are trying to solve a group of scientists from the University of Maryland (UMD), which won a grant from NASA. So how do scientists come to solving all these problems? First, they decided to “attack” one of the main problems of lithium-sulfur batteries, namely, self-discharge. And instead of a liquid organic electrolyte, which, as mentioned above, gradually dissolves the active materials, they used a solid ceramic electrolyte, or rather, Li 6 PS 5 Cl, which conducts lithium ions quite well through its crystal lattice.
But if solid electrolytes solve one problem, they also create additional difficulties. For example, large changes in the cathode volume during the reaction can lead to a rapid loss of contact between the solid electrode and the electrolyte, and a sharp drop in battery capacity. Therefore, scientists have proposed an elegant solution: they created a nanocomposite consisting of nanoparticles of cathode active material (LI 2 S) and electrolyte (Li 6 PS 5 Cl) enclosed in a carbon matrix.

A source
This nanocomposite has the following advantages: firstly, the distribution of nanoparticles of the material, which changes in volume upon reaction with lithium, in carbon, the volume of which practically does not change, improves the mechanical properties of the nanocomposite (plasticity and strength) and reduces the risk of cracking. In addition, carbon not only improves conductivity, but also does not prevent the movement of lithium ions, since it also has good ionic conductivity. A due to the fact that the active materials are nanostructured, lithium does not need to move long distances to react, and the entire volume of material is used more efficiently. Finally, the use of such a composite improves the contact between the electrolyte, the active material, and the conductive carbon.
As a result, scientists received a completely solid battery with a capacity of about 830 mAh / g. Of course, it’s too early to talk about launching such a battery into space, since such a battery works for only 60 charge / discharge cycles. But at the same time, despite such a rapid loss of capacity, 60 cycles is already a significant improvement over previous results, since before this solid lithium-sulfur batteries did not work for more than 20 cycles. It should also be noted that such solid electrolytes can work in a large temperature range (by the way, they work best at temperatures above 100 ° C), so that the temperature limitations of such batteries will be due to active materials rather than electrolyte, which distinguishes such systems from batteries that use organic solutions in the form of an electrolyte.
Sources
Nano Lett., 2016, 16 (7), pp 4521–4527
interface.ecsdl.org/content/25/3/26
interface.ecsdl.org/content/25/3/26
Source: https://habr.com/ru/post/398529/
All Articles