Is it possible to make an edible power source or the principle of operation of an abiotic fuel cell? Part 1: Theory
On the Internet there are many instructions on how to make "edible" batteries from lemons, etc. But the edibility of such devices is questionable, since electrodes, as a rule, are metal plates (usually zinc and copper), and only electrolyte, of which lemon plays the role, is edible. Recently I wondered, is it possible to create a fully edible battery?
Immediately I will explain, the battery should not be from biocompatible, but from edible components. Of course, it would be easy to make an edible supercapacitor of activated carbon, but, as you know, the energy intensity of supercapacitors is very low compared to batteries.
The main difficulty of such an idea is to find suitable electrodes / current collectors. It is necessary, firstly, that the edible material has good conductivity, and secondly, it could participate in electrochemical reactions. And the first thing that comes to mind when you think about edible conductors are food colors E174 and E175, they are food gold and silver. But in what electrochemical reactions can these materials be used if they stand close to each other in an electrochemical series of voltages? But here we must remember that gold and silver possess electrocatalytic activity. And where are catalysts used in electrochemical power sources? In the fuel cell!
How does a fuel cell work? Like other electrochemical power sources, fuel cells have two electrodes immersed in an electrolyte. But unlike batteries and supercapacitors, these electrodes are not active materials, but catalysts, on which oxidation of the “fuel” takes place, which is supplied from the outside, and the oxidizer is reduced. For example, in the classic example of a hydrogen fuel cell, the fuel is hydrogen, and the oxidant is oxygen. By the way, compared with other electrochemical power sources, for example, batteries and supercapacitors, fuel cells are the most energy-intensive, since they work while reagents are supplied to the electrodes. But their disadvantage is low power, limited by the rate of electro-chemical reactions and the rate of supply of fuel and oxidizer to the electrodes.
')
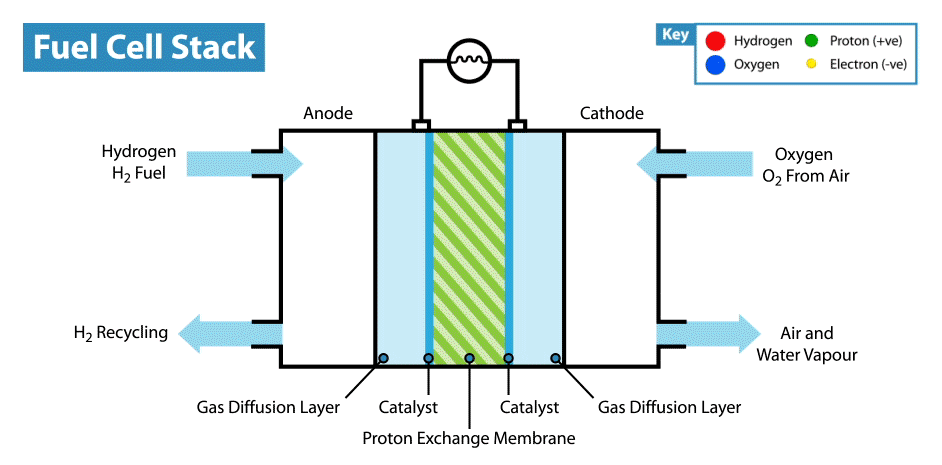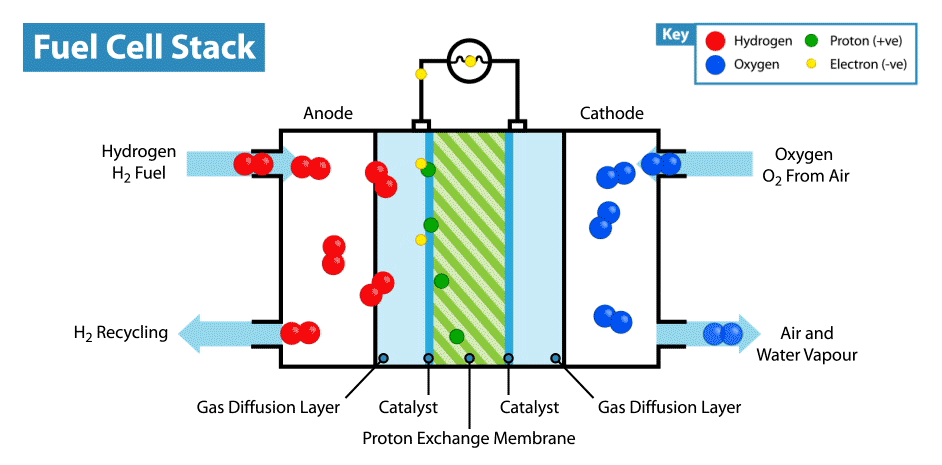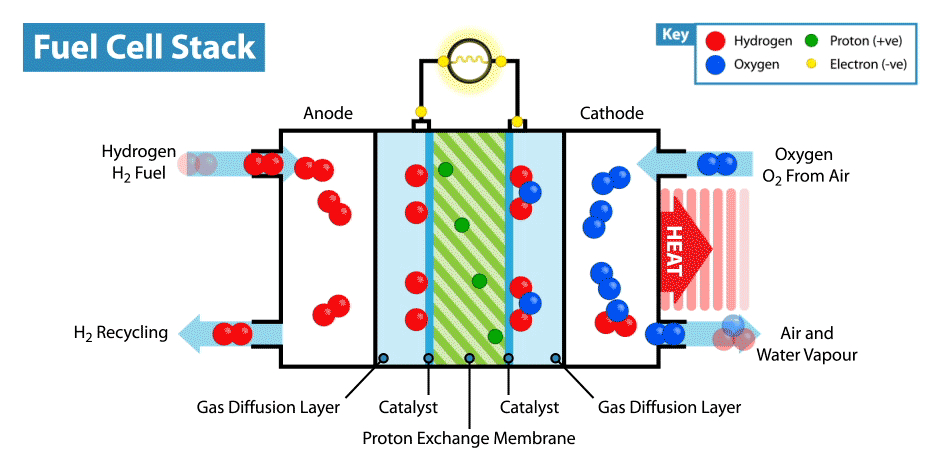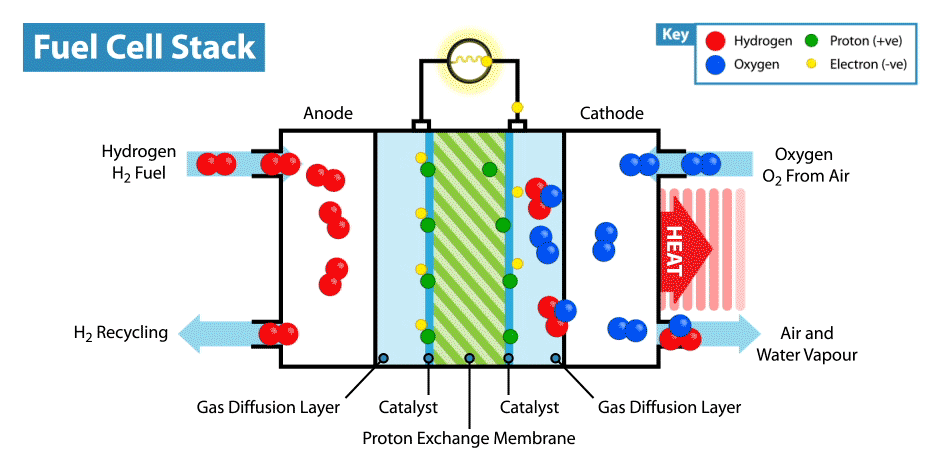
But back to the idea of an edible fuel cell. If oxygen can play the role of an oxidizer, then what can be used as an edible fuel? Searching for fuel cell options in the scientific literature, I came across an interesting solution. It turns out that ordinary glucose can be used in the form of topiva, and this type of device is called an abiotic fuel cell .

The voltage of such cells is usually less than 0.5 V, a couple of tens of microamperes per square centimeter of the electrode, so the phone is not charged with such a device, but at the moment abiotic fuel cells are being developed for use as batteries in biomedical microelectromechanical systems (BioMEMS) that must be implanted into the human body. Why "abiotic"? But the fact is that there are several types of fuel cells that use glucose as fuel: enzymatic, microbial, and abiotic. Enzymatic fuel cells use isolated enzymes to oxidize glucose. But the problem is that the enzymes are quickly deactivated, and such systems usually lose power quickly, which is a big problem for implantable systems. Microbial fuel cells, in turn, use microorganisms, which is also impractical for oral administration due to the risks of infection. Unlike the systems described above, abiotic fuel cells for the processing of glucose are not used by living organisms or biomolecules, but by noble metals.
The ideal design of such a device is as follows: two metal catalysts are immersed in a glucose solution, in which dissolved oxygen is also present. In this case, one of the electrodes is a selective catalyst for the oxidation of glucose, and the other is for the reduction of oxygen. That is, the edible fuel cell could theoretically be made in the form of a sweet jelly, on one side coated with edible gold, which would oxidize glucose, and on the other, silver, which restores oxygen. But will such a device work?
And here we are confronted with a second difficulty in creating an edible fuel cell: most noble metals have catalytic activity both in the oxidation of glucose and in the reduction of oxygen. That is, even if silver is less “sensitive” to glucose and has better catalytic activity for oxygen reduction, the voltage of our cell will be less than 0.5 V, since the oxygen reduction reaction will be parallel to the anode and cathode.
In general, I would like to test these theoretical reflections in practice, so in the next posts we will talk about attempts to construct an edible abiotic fuel cell at home.
Immediately I will explain, the battery should not be from biocompatible, but from edible components. Of course, it would be easy to make an edible supercapacitor of activated carbon, but, as you know, the energy intensity of supercapacitors is very low compared to batteries.
The main difficulty of such an idea is to find suitable electrodes / current collectors. It is necessary, firstly, that the edible material has good conductivity, and secondly, it could participate in electrochemical reactions. And the first thing that comes to mind when you think about edible conductors are food colors E174 and E175, they are food gold and silver. But in what electrochemical reactions can these materials be used if they stand close to each other in an electrochemical series of voltages? But here we must remember that gold and silver possess electrocatalytic activity. And where are catalysts used in electrochemical power sources? In the fuel cell!
How does a fuel cell work? Like other electrochemical power sources, fuel cells have two electrodes immersed in an electrolyte. But unlike batteries and supercapacitors, these electrodes are not active materials, but catalysts, on which oxidation of the “fuel” takes place, which is supplied from the outside, and the oxidizer is reduced. For example, in the classic example of a hydrogen fuel cell, the fuel is hydrogen, and the oxidant is oxygen. By the way, compared with other electrochemical power sources, for example, batteries and supercapacitors, fuel cells are the most energy-intensive, since they work while reagents are supplied to the electrodes. But their disadvantage is low power, limited by the rate of electro-chemical reactions and the rate of supply of fuel and oxidizer to the electrodes.
')

But back to the idea of an edible fuel cell. If oxygen can play the role of an oxidizer, then what can be used as an edible fuel? Searching for fuel cell options in the scientific literature, I came across an interesting solution. It turns out that ordinary glucose can be used in the form of topiva, and this type of device is called an abiotic fuel cell .

The voltage of such cells is usually less than 0.5 V, a couple of tens of microamperes per square centimeter of the electrode, so the phone is not charged with such a device, but at the moment abiotic fuel cells are being developed for use as batteries in biomedical microelectromechanical systems (BioMEMS) that must be implanted into the human body. Why "abiotic"? But the fact is that there are several types of fuel cells that use glucose as fuel: enzymatic, microbial, and abiotic. Enzymatic fuel cells use isolated enzymes to oxidize glucose. But the problem is that the enzymes are quickly deactivated, and such systems usually lose power quickly, which is a big problem for implantable systems. Microbial fuel cells, in turn, use microorganisms, which is also impractical for oral administration due to the risks of infection. Unlike the systems described above, abiotic fuel cells for the processing of glucose are not used by living organisms or biomolecules, but by noble metals.
The ideal design of such a device is as follows: two metal catalysts are immersed in a glucose solution, in which dissolved oxygen is also present. In this case, one of the electrodes is a selective catalyst for the oxidation of glucose, and the other is for the reduction of oxygen. That is, the edible fuel cell could theoretically be made in the form of a sweet jelly, on one side coated with edible gold, which would oxidize glucose, and on the other, silver, which restores oxygen. But will such a device work?
And here we are confronted with a second difficulty in creating an edible fuel cell: most noble metals have catalytic activity both in the oxidation of glucose and in the reduction of oxygen. That is, even if silver is less “sensitive” to glucose and has better catalytic activity for oxygen reduction, the voltage of our cell will be less than 0.5 V, since the oxygen reduction reaction will be parallel to the anode and cathode.
In general, I would like to test these theoretical reflections in practice, so in the next posts we will talk about attempts to construct an edible abiotic fuel cell at home.
Source: https://habr.com/ru/post/398091/
All Articles