What caused the differences between batteries and supercapacitors
Electrochemical power sources are used everywhere today and have distinctive characteristics: capacity, or the amount of stored energy, as well as power, or the ability to quickly give or accumulate this energy (discharge / charge at high currents). In addition, safety and durability are very important for batteries. In this post I will tell you how the batteries and supercapacitors differ at the chemical level, and how this affects their technical characteristics.
I'll start with batteries. Today, lithium-ion and nickel-metal hydride (NiMH) batteries are most commonly used, but lithium-ion batteries are gradually replacing NiMH for several reasons. First, lithium-ion batteries are more energy-intensive. This is because, compared to NiMH alkaline electrolytes, which limit the cell voltage to 1.2 V, carbonate-based electrolytes of lithium-ion batteries provide a voltage of 3V. And this means a smaller number of cells needed to achieve a certain voltage, as well as more compact dimensions, which is simply necessary for modern portable electronic devices. And, most importantly, in comparison with NiMH, where alloys with rare-earth metals are used, lithium-ion batteries contain cheaper materials.
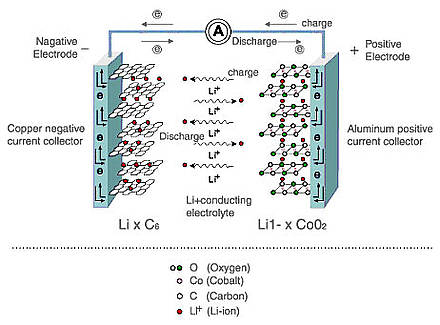
The operation of lithium-ion batteries is as follows: lithium ions are embedded in the layered material of the anode (most often graphite) or cathode (transition metal oxides) during charging and discharging. Capacity is determined by how much lithium is built into the electrodes, and if the capacity, as mentioned above, lithium-ion batteries are good, then with power (this is the ability of the battery to quickly charge and discharge at high currents, for example, during acceleration and regenerative braking in electric vehicles) It is not that simple. For example, when charging too fast, lithium ions do not have time to be embedded in crystals and form chains of metallic lithium (dendrites) on the anode, which can lead to a short circuit, especially at low temperatures. A too fast discharge can destroy the cathode crystal and lead to premature aging of the battery.
What does battery power depend on? Battery power depends on several parameters: the conductivity of the electrode, consisting of the active material and additives, the rate of electrochemical processes taking place in the active materials, and the ionic conductivity of the electrolyte. In order to somehow improve the power of lithium-ion batteries, if they are meant to be used at high currents, manufacturers create special, thinner electrodes: they contain less active material, but more carbon additives. As a result, the conductivity of the electrode increases, but, alas, reducing the amount of active material decreases and the capacitance. Moreover, even if such a technology improves the conductivity of the electrodes, one should not forget about other parameters affecting the power, especially the slow incorporation of lithium into crystals (diffusion difficulties), which this technology does not affect in any way.
')
But here nanomaterials come to our aid: in order to integrate into a nanocrystal, lithium does not need to move over long distances, so the intercalation takes place much faster.
But alas, nanomaterials also have disadvantages, in particular, their increased chemical reactivity, which reduces the battery life. In general, trying to improve one of the battery parameters, often all others deteriorate.
But if the battery still has to work at very high currents, at which neither the method of producing electrodes nor the structuring of active materials help, a supercapacitor comes to the rescue. At first glance, a supercapacitor resembles a battery: it also has two electrodes placed in an electrolyte. But this is only at first glance. In fact, the energy supercapacitor stores in the form of a layer of ions that are attached to the surface of the electrodes (electric double layer). The capacity of such devices directly depends on the electrode surface, and activated carbon is often used as the active material. Since, unlike the lithium-ion batteries, in supercapacitors there are no redox reactions and the ions should not be embedded anywhere, charging and discharging take place much faster, and the devices themselves are more durable.
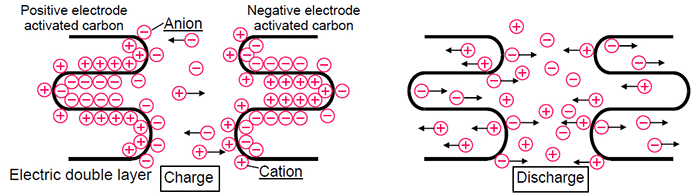
But why, having such remarkable power, supercapacitors cannot be used as independent power sources instead of batteries? But the point here is that the process of formation of a double electric layer is much less energy-intensive than redox reactions, therefore, despite the fact that supercapacitors accumulate energy and give up quickly, its amount is very small compared to batteries. In addition, supercapacitors are subject to strong self-discharge: if a charged battery loses several percent of its capacity in a month, the supercapacitor can be completely discharged during this time. Therefore, supercapacitors are usually used in conjunction with energy-intensive batteries and take on the role of a power source only at peak loads.
Self-discharge is a gradual voltage drop in an electrochemical power source if it is disconnected from the network. In lithium-ion batteries, it is associated with the gradual oxidation of electrolyte at the cathode, which results in the release of electrons that are used by cathode materials to incorporate lithium into their structure (a process that occurs during discharging). Since the electrolyte is oxidized slowly, self-discharge is also slow. But the exact mechanism of self-discharge of supercapacitors is still unknown, but it is associated with electrolyte ions, which enter into redox reactions on the surface of the electrodes.
In the end it should be said that there are also “pseudo-intensive” supercapacitors, also called electrochemical supercapacitors, in which more energy-intensive redox processes take place on the surface of active materials, but the capacity of such devices is still lower than that of batteries, and they also suffer from strong self-discharge.
Sources:
I'll start with batteries. Today, lithium-ion and nickel-metal hydride (NiMH) batteries are most commonly used, but lithium-ion batteries are gradually replacing NiMH for several reasons. First, lithium-ion batteries are more energy-intensive. This is because, compared to NiMH alkaline electrolytes, which limit the cell voltage to 1.2 V, carbonate-based electrolytes of lithium-ion batteries provide a voltage of 3V. And this means a smaller number of cells needed to achieve a certain voltage, as well as more compact dimensions, which is simply necessary for modern portable electronic devices. And, most importantly, in comparison with NiMH, where alloys with rare-earth metals are used, lithium-ion batteries contain cheaper materials.

The operation of lithium-ion batteries is as follows: lithium ions are embedded in the layered material of the anode (most often graphite) or cathode (transition metal oxides) during charging and discharging. Capacity is determined by how much lithium is built into the electrodes, and if the capacity, as mentioned above, lithium-ion batteries are good, then with power (this is the ability of the battery to quickly charge and discharge at high currents, for example, during acceleration and regenerative braking in electric vehicles) It is not that simple. For example, when charging too fast, lithium ions do not have time to be embedded in crystals and form chains of metallic lithium (dendrites) on the anode, which can lead to a short circuit, especially at low temperatures. A too fast discharge can destroy the cathode crystal and lead to premature aging of the battery.
What does battery power depend on? Battery power depends on several parameters: the conductivity of the electrode, consisting of the active material and additives, the rate of electrochemical processes taking place in the active materials, and the ionic conductivity of the electrolyte. In order to somehow improve the power of lithium-ion batteries, if they are meant to be used at high currents, manufacturers create special, thinner electrodes: they contain less active material, but more carbon additives. As a result, the conductivity of the electrode increases, but, alas, reducing the amount of active material decreases and the capacitance. Moreover, even if such a technology improves the conductivity of the electrodes, one should not forget about other parameters affecting the power, especially the slow incorporation of lithium into crystals (diffusion difficulties), which this technology does not affect in any way.
')
But here nanomaterials come to our aid: in order to integrate into a nanocrystal, lithium does not need to move over long distances, so the intercalation takes place much faster.

But alas, nanomaterials also have disadvantages, in particular, their increased chemical reactivity, which reduces the battery life. In general, trying to improve one of the battery parameters, often all others deteriorate.
But if the battery still has to work at very high currents, at which neither the method of producing electrodes nor the structuring of active materials help, a supercapacitor comes to the rescue. At first glance, a supercapacitor resembles a battery: it also has two electrodes placed in an electrolyte. But this is only at first glance. In fact, the energy supercapacitor stores in the form of a layer of ions that are attached to the surface of the electrodes (electric double layer). The capacity of such devices directly depends on the electrode surface, and activated carbon is often used as the active material. Since, unlike the lithium-ion batteries, in supercapacitors there are no redox reactions and the ions should not be embedded anywhere, charging and discharging take place much faster, and the devices themselves are more durable.

But why, having such remarkable power, supercapacitors cannot be used as independent power sources instead of batteries? But the point here is that the process of formation of a double electric layer is much less energy-intensive than redox reactions, therefore, despite the fact that supercapacitors accumulate energy and give up quickly, its amount is very small compared to batteries. In addition, supercapacitors are subject to strong self-discharge: if a charged battery loses several percent of its capacity in a month, the supercapacitor can be completely discharged during this time. Therefore, supercapacitors are usually used in conjunction with energy-intensive batteries and take on the role of a power source only at peak loads.
Self-discharge is a gradual voltage drop in an electrochemical power source if it is disconnected from the network. In lithium-ion batteries, it is associated with the gradual oxidation of electrolyte at the cathode, which results in the release of electrons that are used by cathode materials to incorporate lithium into their structure (a process that occurs during discharging). Since the electrolyte is oxidized slowly, self-discharge is also slow. But the exact mechanism of self-discharge of supercapacitors is still unknown, but it is associated with electrolyte ions, which enter into redox reactions on the surface of the electrodes.
In the end it should be said that there are also “pseudo-intensive” supercapacitors, also called electrochemical supercapacitors, in which more energy-intensive redox processes take place on the surface of active materials, but the capacity of such devices is still lower than that of batteries, and they also suffer from strong self-discharge.
Sources:
Linden's Handbook of Batteries, Fourth Edition
IEEE TRANSACTIONS ON POWER ELECTRONICS, Vol. 24, N ° 2, 2009
J. Electrochem. Soc., Vol. 145, N ° 10, 1998
BE Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, 1999
Source: https://habr.com/ru/post/397915/
All Articles