Ask Ethan # 92: Is there a limit on the maximum temperature?
If you take all the energy out of something, you can achieve absolute zero, the coldest temperature. But is it possible to achieve the highest?
Nothing is lost, everything is only transformed.
- Michael Ende
At the end of each week, we choose one of the questions sent to answer it. This week, the honor goes to school teacher Cameron Peters, who asks:
I teach science the 8th grade and my students pass the concept of temperature. In particular, we considered the concept of absolute zero, what it means and how it relates to the movement of atoms. My students want to know whether there is a maximum temperature attainable in nature, or whether there is no upper limit.
Let's start with the positions that should be known to the eighth-grader, and we will gradually increase the degree.
')
Take the classic experiment: dissolving food coloring in water of different temperatures. What will we see? The higher the temperature, the faster the dye will dissolve.
Why it happens? Because the temperature of the molecules is directly related to the kinetic motion — and speed — of the particles. This means that in hotter water, individual molecules move faster, and that the dye particles scatter by volume of hot water faster than by cold water.

If we completely stopped all this movement - and everything would stop (and even overcome the nature of quantum physics) - this would allow you to reach absolute zero: the lowest thermodynamic temperature.
But what about the opposite direction? If you heat up a particle system, they will simply move faster. But is there a temperature height limit, and will you face a catastrophe preventing you from rising higher? Let's get a look.
At temperatures of thousands of kelvins, the heat transferred to the molecules will begin to destroy even the bonds that hold them together, and if you continue heating, the electrons will begin to break away from the atoms. You will get an ionized plasma consisting of electrons and atomic nuclei, without any neutral atoms.
But this is still permissible: the individual particles - electrons and positively charged ions - will perfectly rebound from each other, and obey the usual laws of physics. And you can still raise the temperature and see what happens next.

And then the individual particles will begin to disintegrate.
• About 8 * 10 9 (8 billion K) of particle collision energies will begin to spontaneously appear matter / antimatter vapors - electrons and positrons.
• About 2 * 10 10 (20 billion K) atomic nuclei will be divided into protons and neutrons.
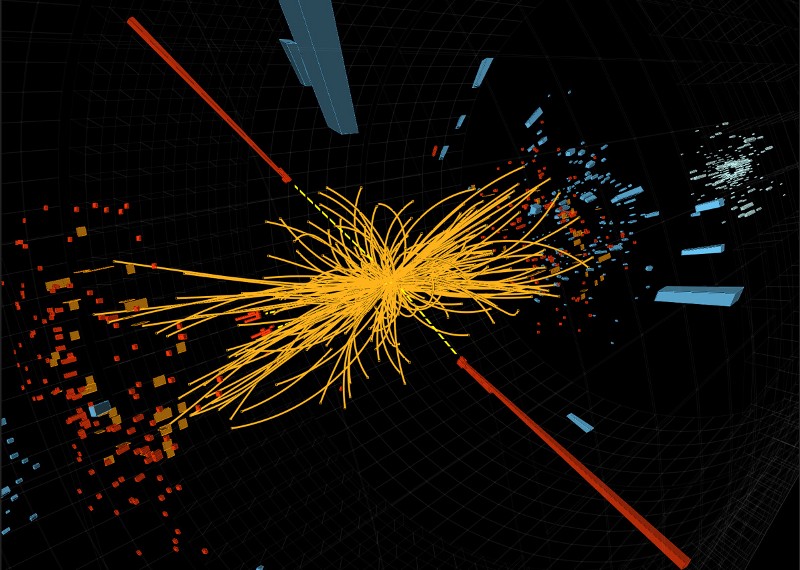
• About 2 * 10 12 (2 trillion K) protons and neutrons will cease to exist, and their constituent particles, quarks and gluons will fly instead of them
• About 2 * 10 15 (2 quadrillion K) all known particles and antiparticles will begin to appear in large quantities.

But this is not the upper limit, not at all. Just at temperatures of the order of 2 * 10 15 (2 quadrillion K) something interesting will start to happen. This is precisely the energy that is needed for the appearance of the Higgs boson, and, therefore, to restore one of the most fundamental symmetries of the Universe: symmetries that give particles a rest mass.
In other words, when you warm up the system even more, you will find that all your particles have lost mass, and fly at the speed of light. And instead of a mixture of matter, antimatter and radiation, everything around will behave like radiation, be it really matter, antimatter, or none of them.
But we are not finished. It is possible to further increase the temperature of the system, and although nothing will move faster inside, it will become more energetic - just like radio waves, microwaves, visible light and X-rays are forms of light (and move at the speed of light), although they all have energy different.
Perhaps there will be some particles that are still unknown to us, or new laws (or symmetries). You might decide that you can move on, to infinite energies.
But there are three reasons why this is impossible.

1) The Universe contains a finite amount of energy. Let us take everything that exists in the foreseeable space-time: all the matter, anti-matter, radiation, neutrinos, dark matter, and even the energy of space itself - and this is a lot. There are about 10 80 particles of normal matter, 10 89 neutrinos and antineutrinos, a little more than photons, and all the energy contained in dark matter and dark energy scattered over a radius of 46 billion light years in the observed Universe around us.
But even if you turn all this into pure energy (through E = mc 2 ), and even if you use all this energy to heat the system, you will not have an infinite amount of energy. If you put all this in one system, there will be a lot of energy, it will correspond to temperatures of about 10 103 K, but this is not infinity. So the upper limit is. But something will stop you even before you reach this state.

2) If you inject too much energy into an enclosed space, you will create a black hole! You imagine black holes as huge, massive and dense objects capable of swallowing huge crowds of planets just like a cookie monster swallows a box of cookies - awkwardly, without difficulty and without hesitation.

But if you give a separate quantum particle enough energy - even if it is a massless particle, moving at the speed of light - it turns into a black hole! There is a scale according to which something, having accumulated enough energy, will not be able to perform interactions, as ordinary particles do. And if you let the particle reach that energy, equivalent to 22 µg according to E = mc 2 , you can reach as much as 10 19 GeV before the system refuses to warm up further. You will spontaneously appear black holes, which then immediately disintegrate to a state of low energy through thermal radiation. So at this energy stage, the Planck energy, there is an upper limit for our Universe, which corresponds to a temperature of “only” of 10 32 K.
It is much smaller than the previous one, since not only the Universe is finite, but black holes also become the limiting factor. But there is one more factor, and I would worry about him first of all, raising the temperature to unmeasured heights.
3) With a certain heat, you restore the potential that caused our Universe to experience inflation. Before the Big Bang, the Universe was in a state of exponential growth, when space itself expanded like a ball, with an exponential speed. All particles, antiparticles and radiation inside it were quickly separated from other pieces of matter and energy, and at the end of inflation, the Big Bang began.
If you reach temperatures sufficient to bring this field to an inflationary state, you will press the reset button of the Universe and force inflation to resume, which will lead to a re-start of the Big Bang.
If this is too difficult for you, remember this: if you manage to raise the temperature to the level required for such an effect, you will not be able to survive. Theoretically, it is estimated at 10 28 - 10 29 KK, although there is a fairly large variation, depending on what step inflation occurs.
Therefore, to rise to very large temperatures is quite easy. And although the physical phenomena familiar to you will differ in details, you will be able to raise the temperature higher and higher, but only to the point after which you destroy absolutely everything that is dear to you. So be careful, Mr. Peters students, but do not be afraid of the Large Hadron Collider. Even in the most powerful accelerator of the Earth, we achieve energies of at least 100 billion times less than the risky ones. Send me your questions and suggestions for the following articles.
Source: https://habr.com/ru/post/397511/
All Articles