Death alternatives: how to beat aging

How does modern molecular biology look at the phenomenon of aging? How are they trying to study aging? Are there any hopes for slowing down or even stopping this process? A lecture by biologist Alexander Panchin was devoted to these issues, with which he spoke at the Set Up popular science lecture center in the Mail.Ru Group office.
Aging is a decrease in life expectancy with age. From a certain age, the probability of death of a person increases every year. After 20 years, every eight years of life increases the risk about two times. People die of heart disease, cancer, stroke, emphysema, pneumonia, kidney disease, Alzheimer's disease, and accidents. But the good news is that scientific and technological progress has already allowed many diseases to be conquered and a person’s life expectancy increased.
Over the past 60-plus years, life expectancy has increased in all countries, with some people living about 20 years longer. In Australia, Canada, Japan, in some European countries, the average life expectancy exceeds 80 years. This is the question of the importance of good medicine in the country, the rational use of scientific advances, the use of effective drugs, and not some homeopathy, and so on. But in general, progress is noticeable around the world.
')
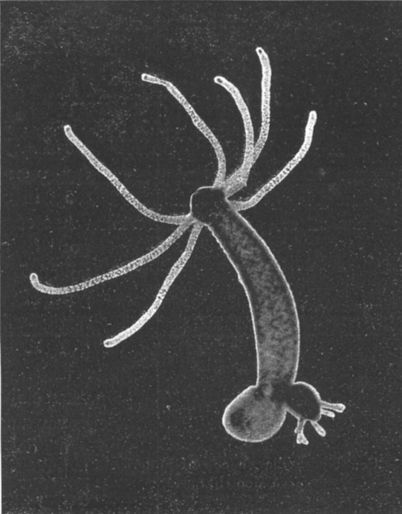
At the end of the XIX century a hypothesis was put forward on the theoretical immortality of the hydra. In 1997, Daniel Martinez proved the hypothesis experimentally.
When the question arises, can we beat this growing schedule of increasing mortality, I recall the words of the Wright brothers: “If birds can fly, then we can achieve a controlled flight.” Biologists say that if the hydra 's risk of death does not increase with age, then we can probably achieve this. There are no fundamental restrictions that prevent a living organism from living for a very long time.
The classic example of a long-lived organism is a naked digger who lives ten times longer than its relatives — mice and rats. Scientists are already exploring the genome of the naked digger in the hope of understanding what makes this creature so special and long-lived.
If we evaluate the diversity of life on a global scale, then mammals may have a life expectancy of almost a hundred times, and in animals as a whole, tens of thousands of times. With what it can be connected? Obviously, genes play an important role in the process of aging and longevity. Any shrew or vole from an evolutionary point of view is not particularly interested in living long. In nature, few of the representatives of these species live to an advanced age; predators eat them quite early. Even if there was some kind of mutation that prolongs life, then it would be of little use in terms of increasing reproductive success. And in species that have got rid of the threat from predators, we are just seeing an increase in life expectancy. The same bare digger living under the ground can live for 31 years. Another example of protection against predators is the ability to fly, as is the case with bats and birds. People can defend themselves with the help of science and technology.
There are organisms whose genome already contains death programs. For example, in some species of salmon, females die immediately after spawning. But there is a more interesting example - this octopus:
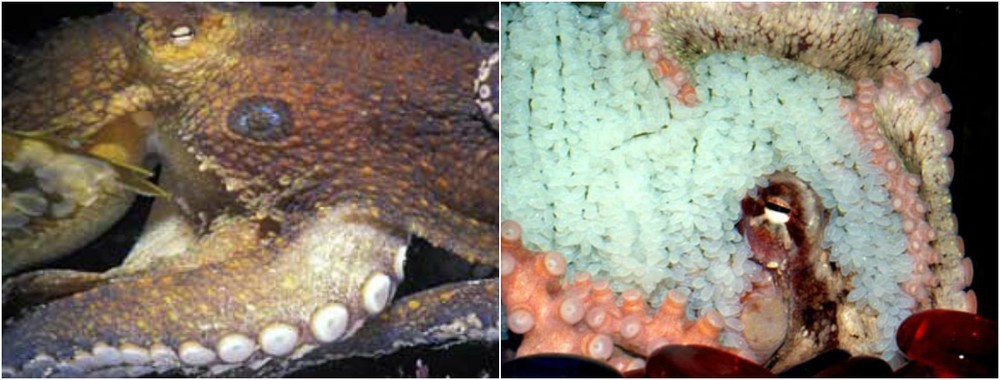
Illustration: A SNAIL'S ODYSSEY
The female octopus lays eggs, stops feeding and dies on average in a month. This is a programmed death. But it turned out that this program can be canceled, moreover, surgically - just cutting a couple of glands, after which the octopus can live not a month, but 6–9 months.
There are examples of programmed cell death, in which individual cells can kill themselves. This process is called apoptosis : when a cell accumulates a large number of mutations, in order not to become cancerous, not to create a threat to the body, it destroys itself, and reasonably enough - the cell does not just splash out all its contents outwards, but collapses into compartments that can then be captured by neighboring cells and disposed of.
It is interesting that there is a transition from the death of cells to the death of the whole organism. The gut of the Caenorhabditis elegans roundworm ( nematode ) fluoresces slightly. And when the worm dies due to natural causes or as a result of some damage, then an hour before death in the front part of the intestine the luminescence increases and the wave gradually passes to the end of the intestine. After that, the worm moves away to another world. This phenomenon is called the "blue wave of death." A wave can be induced artificially, for example, by freezing and thawing a worm. Also, scientists have found a way to stop this wave. The life span of a worm does not increase, but if it is damaged, it does not die from it. With the appearance of a wave, the process of cellular death starts, but then this blue glow fades away, and the worm continues to live on. The luminescence itself is just an indicator, but in reality it is a wave of cell death along the intestine, which leads to death.
Why did this mechanism arise? Apparently, as a result of such a “death in an hour,” the worm goes as food to its own descendants. Worms carry babies, die and become food for the next generation. The symbol of sacrificial motherhood.
In humans, at least in adults, no such mechanisms have been found. In our country, aging is a combination of many different processes. One of them is cell aging.
Older cells can damage adjacent ones, can secrete substances that lead to inflammation. Old cells can become cancerous, and therefore it would be good to get rid of them. Recently, the results of studies on the removal of old cells of a certain type in genetically modified rodents with a special drug have been published. And these rodents lived longer. That is quite realistic to find an approach to aging cells.
Telomere shortening
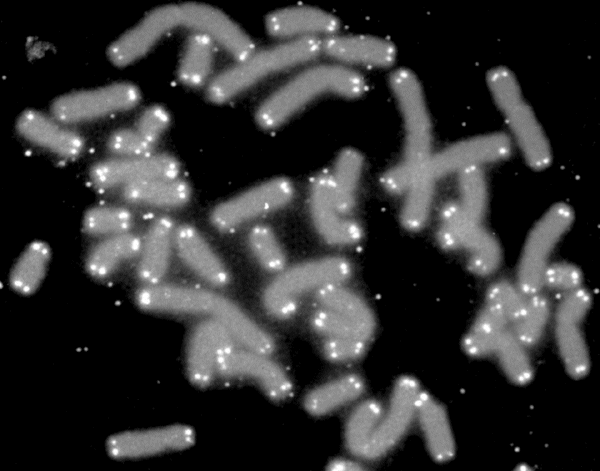
Human chromosomes are gray, telomeres are white
Cell aging is associated with certain processes. One of them is telomere shortening. This is the nature of nature that with each cell division, the DNA molecule is shortened. At the ends of the chromosomes there are areas called telomeres, which are subject to shortening, thereby protecting the rest of the chromosome. Telomeres are shortened from generation to generation, and in old cells they become very short. That is, the cells of the old and young man can be distinguished by the length of the telomeres, taking into account some variations in these characters.
However, the shortening problem has already been solved in our stem cells. They have the enzyme telomerase , which is able to complete the construction of telomeres. Therefore, some cells are said to be immortal.
Another great news is that we can lengthen telomeres with the help of gene therapy. Experiments were carried out in which telomerase was delivered to rodents using a special virus, and telomeres were expanded in cells. Such rodents, as experiments show, live longer.

44-year-old Elizabeth Parrish, head of the American research company BioViva, may have become the first person to successfully test rejuvenating gene therapy.
These experiments inspired Elizabeth Parrish to try this kind of gene therapy on herself. It is clear that she does this as part of a PR campaign, because she owns a large biotechnology company. There was information in the news that it seemed like her telomeres were lengthening. But this is not really a scientific experiment, there is no control group, nothing to compare. Although this story is inspiring by many, rodent experiments suggest that telomere shortening is not an insurmountable problem of aging.
Damage accumulation
Another thing that happens in cells is the accumulation of damage. They are different. One type of damage is the accumulation of all kinds of garbage in the cells. For example, poorly folded proteins, as is the case with Alzheimer's disease . Or improperly working organelles like spoiled mitochondria . With each cell division, these injuries are diluted, as it were, and each of the two resulting cells accounts for two times less. That is, with active division, the cells could rejuvenate.
But the division has a dark side - cancer. There are genetic mutations that are not diluted. A cancer cell is a cell that has accumulated a large number of mutations that can lead to unlimited division, disabling apoptosis. Today, there are more and more modern methods aimed at fighting cancer. Previously, he was a completely incurable disease, now in some cases it is treated.
Starvation
One of the most beautiful life extension experiments was associated with the study of the effects of partial starvation. The experiment was conducted on rodents. This is a mortality chart:
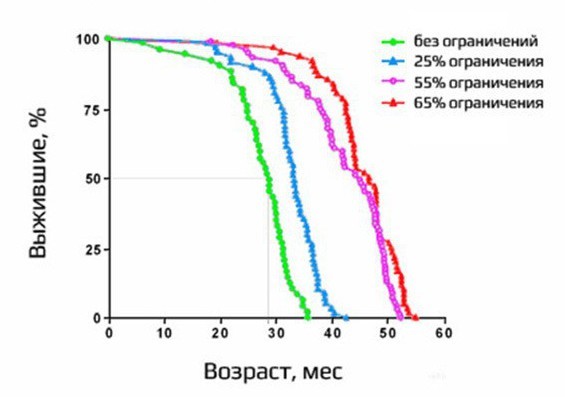
Green color shows those who ate from the belly, blue spruce is 25% less, purple - 55% less, and red - 65%. As you can see, the maximum lifespan increased about 1.5 times. The greens died about three years old, and the rest lived significantly longer. When all the greens have already died, the red ones are still alive. Impressive result. It turned out that the mechanism of life extension through starvation is relevant for very different organisms, although not for everyone. There are those whose partial fasting shortens their lifespan. But for many species it works. For example, the roundworm Caenorhabditis elegans, rats, some strains of mice, dogs, have conflicting data regarding macaques. It is very difficult to put such experiments on people.
When they found out how partial starvation prolongs the life of a worm, they found hormones in it that are released in response to food intake. These hormones act on the DAF-2 receptor, located on the cell surface, and this somehow contributes to aging. If this receptor is spoiled, then such worms live about twice as long.
When the DAF-2 receptor is activated, it starts a cascade of cellular processes, leading to inactivation of a protein called DAF-16. DAF-16 is a very important protein that regulates cell function as follows. If the food is available and DAF-16 is inactivated, the cell thinks: “Oh, zashib, the food is full, everything is fine, you can relax, nothing threatens me, you can multiply, share - everything is fine.” If this signal is not present, that is, DAF-2 is corrupted, or if there is no food, then DAF-16 is activated, the cell thinks: “What a horror, I die, stress, nightmare! You need to save yourself! ”- and launches a bunch of protection mechanisms: from mutations, from oxidative stress, heat shock, just in a row. That is, DAF-16 makes hundreds of other genes work that protect the cell from various problems.
It turned out that humans have proteins similar to DAF-16 and DAF-2. But a mutation was found in a similar to DAF-16 protein. There are people with one variant of this protein, and there are people with another option. The owners of the first variant ( FOXO3 ) live up to 90 years and more often. That is, studying the molecular mechanism of aging in worms, we better understand how the mechanisms of aging in humans are arranged.
Of course, not everything that we learn about worms can be broadcast to people. For example, if we remove the sex cells from the gonad of the worm, this also activates DAF-16 and it will live longer. But if you castrate yourself, this will not prolong your life.
Body mass
Pygmy bats can live up to 40 years. Higher weight organisms live longer. But there are those whose life expectancy is much higher than estimated on the basis of their body weight. For example, a person lives much longer than other species with the same mass. Dwarf bats and their relatives also belong to long-livers. These bats showed a protein similar to DAF-2, but with specific mutations.
In addition to model organisms, scientists are exploring and mutations that occur in humans.

This young man died at the age of 17, he had a syndrome of premature aging, called progeria . This is a genetic disease that causes the early signs of aging. At a young age, senile diseases arise, and such people live on average 13 years. A mutation in the gene that is responsible for protein synthesis Lamin A leads to this disease. This protein is responsible for the organization of the nucleus, the so-called nuclear matrix, the structure of the nucleus. In worms, with age, the nuclear matrix is destroyed in cells, and the slower this happens, the longer the worms live. Perhaps the destruction of the nuclear matrix plays an important role in human aging.
A growth hormone
Growth hormone indirectly leads to signal amplification, which is transmitted through our analogue DAF-2. If greatly simplified, then the dwarf cells feel some effect of starvation. Here's a dwarf little mouse Yoda and his girlfriend Princess Leia:

At Yoda, the gene regulating the synthesis of growth hormone was spoiled. He lived in the laboratory for four years - in terms of human age, this is more than a hundred years.

In Ecuador, there is a population of people with a certain form of dwarfism, which is called Laron syndrome , there are about a hundred people. We do not know yet how much they live. Scientists have been observing them for more than 20 years, and there has been a publication that these people have reduced the risk of certain senile diseases, including cancer and diabetes. Maybe they really live longer compared to other residents of the same region.
God tor the master of garbage
Illustration: The Ban Lab
Our cells are able to remove garbage from themselves, not only during division. They can absorb it. This is called autophagy - the cells digest all the nastiness inside themselves, then it is somehow disposed of and discarded. This process may contribute to cell rejuvenation, but TOR protein prevents it.
What is he doing? He says: “Everything is useful. This rotten sandwich that you have here lies in handy. It will be hungry, there will be a blockade, and it will go into action. ” Our ancestors lived in conditions of a regular shortage of food. There were no genetically modified organisms, there was no green revolution, normal agriculture, there was nothing. Hunger could come anytime, and therefore TOR played an important role. But now this garbage accumulates and damages cells. Fortunately, there are inhibitor substances that can crush this protein.
TOR inhibitors trigger autophagy through this protein. TOR itself is named after rapamycin: Target Of Rapamycin (rapamycin target). Rapamycin is an antibiotic that is produced by mushrooms, it is also used as an immunosuppressant. This substance is used in organ transplantation to reduce the risk of rejection. Rapamycin also prolongs the life of different organisms, in particular mice, and quite strongly.
Rapamycin is very expensive. But there is a cheap inhibitor TOR - cofenin. Animal studies have confirmed that it improves autophagy and prolongs life. There are also the results of epidemiological studies, according to which people who drink several cups of coffee a day, mortality is reduced by 10%.
There is the concept of "French paradox": the French live long and rarely suffer from cardiovascular diseases. At one time this was explained by the consumption of resveratrol contained in red wine. To rodents this substance prolonged life. But then they counted how much a person should consume wine, so that resveratrol had the same effect - two barrels per day. Apparently, the French paradox is most likely associated with ethanol itself, which in a certain small concentration extended the life of nematodes. People who drink a glass of wine per day also reduce the risk of cardiovascular diseases, such as coronary heart disease, coronary heart disease, and so on. But ethanol has negative side effects: alcoholism, cirrhosis of the liver, the risk of certain cancers increases. Therefore, can ethanol be recommended as a geroprotector? This is a controversial issue.
It is of interest to scientists and metformin - a cure for diabetes. It has some side effects associated with the gastrointestinal tract. There is a study confirming that metformin prolongs the life of worms.
But the longest - by 50% - nematodes live thanks to alpha-ketoglutarate . It is remarkable in that it is present in humans in large quantities and has low toxicity. But research on rodents has not yet been conducted, because it is very difficult for scientists to obtain funding for the study of this medicine for aging, because aging is not considered a disease. Alpha-ketoglutarate, by the way, athletes use as a food additive to accelerate the growth of muscle mass. But there is very conflicting evidence whether it really helps in this.
Young blood
Illustration: Popular Science
In one study, scientists surgically connected the bodies of two rodents, the old and the young, uniting their blood systems. Under these conditions, the old rodent lives longer, and the young - less, while the old rodents increase the plasticity of the nervous system, that is, the ability of nerve cells to form new nerve connections. They even improve the regeneration of muscle tissue. It is clear that no one offers to stitch together pensioners and small children so that the pensioners live longer. But maybe we will find some factors that are present in the young blood plasma, or we will find cells that prolong life, and we will be able to synthesize them and give them to older people as a medicine. Tony Wyss-Coray told this remarkably well. Technical tests are being carried out on the use of plasma of young organisms for the treatment of Alzheimer's disease. Let's see what they do.
Live long

Another story is related to researcher Aubrey de Gray, who also spoke at Ted Talk . Aubrey said: “Some people say:“ Well, we will extend life for 20 years, die not at 80, but at 100. But this is still not very comforting. Can we count on something more? "". Aubrey replied, "Yes, we can." Why? He leads a metaphor with a worldwide aggression. If you take the ball and throw it, it will fall. If you throw it strongly, it will fall a little later. But if you throw it in such a way that it reaches the second cosmic velocity, the ball will fly off into space and will never return to Earth. "Look, scientific and technical progress is developing faster and faster, and maybe in the 20 years that you still live, they will come up with a drug that prolongs life by another 25 years, and during those 25 years they will come up with a drug that prolongs life for another 30 years, and so on. And maybe, from a certain age, some people will be able to live for a very long time, thousands of years. ” Many scientists are skeptical. But there are no serious arguments why this is impossible in principle.
We see that scientific and technical progress extends the life of people, and all over the planet, in all countries, even in the poorest, and the life expectancy is steadily increasing. And this means that in order to prolong life, it is necessary to develop science.
Finally, I would like to recommend the wonderful site geroprotectors.org - there are collected publications about the results of research of various substances from the point of view of extending the life of experimental animal organisms.
Source: https://habr.com/ru/post/394907/
All Articles