Frozen time. Scientific approaches to dating

Today's story will be about the methodology of science, in particular about how we can determine the age of archaeological finds, what basic methods are used and what physical principles and processes underlie them.
The beauty of scientific dating methods is that they are complementary and mutually verifiable, that is, with the help of one method we can verify the correctness of the other and vice versa, if necessary introducing amendments to it. Also, these “clocks” cover a huge time range - about 9 orders of magnitude (actually more, but “fast” clocks are useless for historical purposes, the scale of evolutionary time takes seven or eight orders of magnitude).
This can be compared with the work of late criminologists, where there are no direct witnesses from the "crime scene", and found only his tracks.
There are many things in science that are beyond the reach of direct observation. This is one of the reasons for mistrust and resistance to science at the "everyday" level. Today, despite the ever-widening knowledge gap between “scientists” and “ordinary people”, it is necessary to make considerable efforts so that people do not have impressions a la “these scientists cannot explain anything themselves, because they use data from the same scientists who took this data from the ceiling. " Unfortunately, this is the opinion that exists outside of science, in particular, among the numerous "disprovers" of history - somewhere in the kitchen or in the garage. Of course, doubts in science are useful, because any theory that is claimed to be scientific, must be fundamentally falsifiable. The trouble is that in order to question the methods described below, it is necessary to falsify facts from biology, physics, geology, archeology, history and chemistry.
All clocks can be divided into two categories - counting (for example, oscillations of a pendulum or a quartz crystal in a household clock) - or measuring (for example, the time of some non-cyclic processes). And those and other hours in some (fortunately, we need) moments can either "reset" or stop, fixing events. Let's start with the "fastest" hours.
Dendrochronology.

At the scale we need, for example, historical, the counting clocks used in dendrochronology — the annual rings of trees — are very convenient. For example, they can be used to determine in which year the tree was cut down, which was used to build a house or a cult building several centuries ago (in fact, there is a continuous dendrochronological scale of about 11,500 years).
How does this method work? Many people know that to determine the age of a newly felled tree, it is necessary to count the rings in its trunk, considering the present ring as the outer ring. Rings reflect changes in growth rate in different seasons of the year - in summer or winter, in the dry season and in the rainy season, and are particularly pronounced in high latitudes, where there is a strong difference between the seasons. At the same time, to determine the age is not required to cut down a tree. You can drill a hole to the middle of the tree and remove the sample. But a simple counting of the rings will not show in which century the log from your house or the mast of your ship was alive. If you want to date a long dead wood, you have to look at the characteristic pattern of rings. Just as the very presence of rings means annual cycles, some years are worse than others, because the weather changes every year: a drought will slow the growth rate, and a rainy year will speed it up; there are cold and warm years, and even years of El Niño or Krakatau eruptions. Years with poor climatic conditions for wood produce narrower rings than good ones. And the pattern of narrow and wide rings in a particular region, created by a specific sequence of different years, is a characteristic “imprint” that accurately marks the years of formation of these rings, recognizable from tree to tree. In addition, you can always take a sample of the material from the desired ring for radiocarbon dating (see below).
All this, of course, is good, but rare of the trees that have fallen asleep were alive in Petrovsky times, let alone the Bronze Age or earlier. There are trees that live for millennia, but most of them are cut down to wood when they are not even a hundred years old. How is the reference collection of rings created for more ancient times? I think you guessed it.
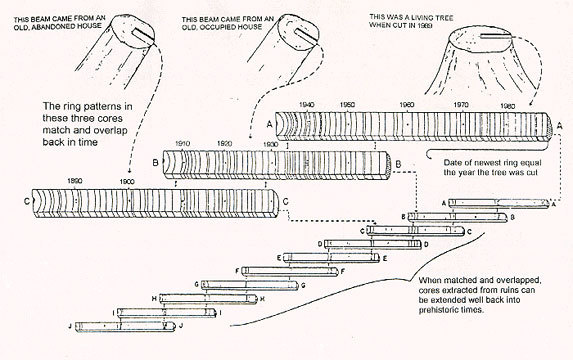
Overlaps. The rope may be one hundred meters long, but the individual fibers in it are much shorter. To use the principle of overlap, you take reference samples of patterns, the date of which can be set on modern trees.
Then you are looking for a pattern of old rings of modern trees and determine the pattern matching among the younger rings of long dead trees. Then identify the pattern of the old rings of these long dead trees, and look for the same pattern in the young rings of even older trees, etc. In practice, this method is used only in archaeological terms, on a scale of several thousand years.
By the way, this is not the only system that promises accuracy up to a year. Sedimentary layers are deposited in glacial lakes. Like the annual rings, they change seasonally and theoretically, the same principle can be used here, with the same degree of accuracy. Corals also have tree rings, like trees. They were used to determine the dates of ancient earthquakes. Most of the other dating systems available to us, including all radioisotope methods, are accurate only within the margin of error, which is proportional to the scale of the measured time.
Radioisotopes
For those who have successfully managed to forget physics, first I will tell basic information about the structure of matter and what radioisotopes are, since here we are dealing with physical processes.
All matter consists of elements that chemically interact with other elements. In nature, there are 92 elements minus technetium, a little more, if we assume artificially synthesized elements. The atomic theory of the structure of matter, which I think even creationists accept, tells us that elements consist of characteristic atoms, which are the smallest particles into which an element can be divided so that it does not cease to be this element. What does an atom look like, like nitrogen, or copper, or carbon?
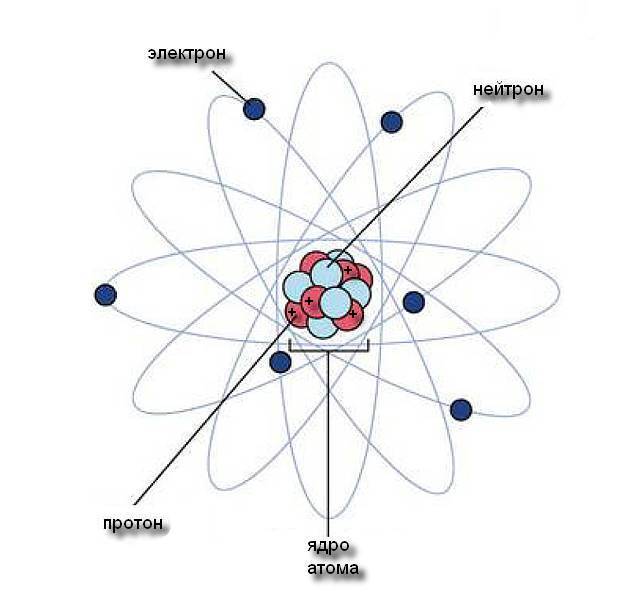
The composition of the atom consists of three kinds of particles, at least in the Bohr model. We are already familiar with electrons. Two other particles, much larger, are called protons and neutrons, and they are in the nucleus and their size is almost the same. The number of protons is constant for any particular element and is equal to the number of electrons. This number is called the atomic number and is written in the subscript next to the name of the element. This is a unique feature of the element, and there are no gaps in the atomic numbers of the famous periodic table of Mendeleev. Each number in it corresponds to exactly one and only one element. The element with atomic number 1 is hydrogen, 2 is helium, 3 is lithium, and so on, up to 92 in uranium.
Protons and electrons carry an oppositely charged electrical charge. We call one of them positive and the other negative, in accordance with arbitrary agreement. These charges are important in the formation of chemical bonds of elements with each other, mainly through the interaction of electrons. Neutrons in an atom are bound to protons in the nucleus and have no charge, and they do not participate in chemical reactions. Neutrons, protons and electrons in any element are exactly the same as in any other. There is no such thing as an oxygen proton, or a potassium electron, or a copper neutron. A proton - it is a proton everywhere, but it makes a copper atom a copper with exactly 29 protons (and 29 electrons) in it. What we think of in our everyday sense as copper is a matter of chemistry. Chemistry - the dance of electrons. All its essence lies in the interaction of atoms through their electrons. Chemical bonds are easily destroyed and re-created because only electrons are separated or exchanged in chemical reactions. The forces of attraction within atomic nuclei are much more powerful. That is why the "splitting of the atom" sounds so ominous, but it can occur in "nuclear" (unlike chemical) reactions, and radioactive clocks are based on them.
Electrons have an insignificant mass, thus the total mass of the atom, its “atomic mass”, is equal to the total number of protons and neutrons. As a rule, it is slightly more than twice the atomic number, because usually in the nucleus, as a rule, there are more neutrons than protons. The atomic mass is written in superscript near the designation of an element in the periodic table. Unlike the number of protons, the number of neutrons in an atom is not a unique feature of an element. Atoms of any particular element can be in different "versions", called isotopes, differing in the number of neutrons, but always with the same number of protons. Some elements, such as fluorine, have only one naturally occurring isotope. The atomic number of fluorine is 9, and its atomic mass is 19, from which it is clear that it has 9 protons and 10 neutrons. Other elements have several isotopes. Lead has five common isotopes. They have the same number of protons (and electrons) - 82, which is the atomic number of lead, but with different atomic masses - from 202 to 208. Carbon has three isotopes found in nature. Carbon-12 is ordinary carbon with the same number of neutrons and protons — by 6. There is also carbon-13, too short-lived for our purposes, and carbon-14, which is rare but not enough to be useful for dating organic samples.
The next important theoretical fact is that not all isotopes are stable. Lead-202 is an unstable isotope, and lead-204, -206, -207, and -208 are stable. “Unstable” means that atoms spontaneously break up into something else, at a predictable rate, albeit at unpredictable moments. The predictability of the decay rate is the key to all radiometric clocks. The synonym for the word "unstable" is "radioactive." There are several types of radioactive decay, suitable as a clock in which neutrons are involved. In one form (β - decay), the neutron turns into a proton. This means that the atomic mass remains the same (protons and neutrons have the same mass), and the atomic number increases by one, so the atom becomes a different element, one cell to the right of the periodic system. For example, cesium-55 is converted to barium-56. With another form of radioactive decay (β + -decay), on the contrary - the proton turns into a neutron. The atomic mass again remains the same, but this time the atomic number decreases by one, and the atom becomes the next element to the left of the periodic system. The third type of radioactive decay ( electron capture ) has the same result. A proton is able to capture one of the electrons of the shell of its atom and turn into a neutron (emitting a neutrino). Again, there is no change in the atomic mass, the atomic number is reduced by one, and the atom turns into the next element to the left in the periodic system. There is also a more complex form of decay, in which the atom emits a so-called alpha particle. It consists of two neutrons and two protons "glued together" (or the nucleus of a helium atom without electrons). This means that the atomic mass is reduced by four, and the atomic number is reduced by two. The atom turns into the element that is two cells to the left in the periodic table. An example of alpha decay is the transformation of the very radioactive isotope of uranium-238 (with 92 protons and 146 neutrons) into thorium-234 (with 90 protons and 144 neutrons).
Now to the point. Each unstable isotope decays at a precisely known rate, for each isotope of its own. In all cases, the decay is exponential. The generally accepted measure of decay rate is the “half-life”. This is the time spent on the decay of half of its atoms. The half-life is the same and does not depend on how many atoms have already decayed. For example, the half-life (T½) of carbon-14 is 5,730 ± 40 years. For 2010, the maximum age of a sample that can be accurately determined by the radiocarbon method is about 60,000 years, that is, about 10 half-lives of 14 C. During this time, the content of 14 C decreases by about 1,000 times (about 1 disintegration per hour per gram of carbon) and we will have to turn to slower hours.
')
Potassium-argon method
An isotope often used on an evolutionary time scale is potassium-40 with a half-life of 1.26 billion years, and it will be used as an example to explain the whole idea of radioactive hours. This “clock” is called potassium-argon, since argon-40 (it is one cell to the left of the periodic system) is one of the elements into which potassium-40 decays (the other, as a result of another type of radioactive decay, is calcium-40, located on one to the right in the periodic system). If we start with a certain amount of potassium-40, then after 1260 million years half of the potassium-40 will decompose into argon-40. This is called the half-life. In another 1.26 billion years, half of what remains (1/4 of the original) and so on will fall apart. Over a period of time shorter than 1.26 billion years, respectively, a smaller amount of the initial potassium will decay. Suppose we have some amount of potassium-40 in a closed system, without argon-40. After several hundred million years have passed, the scientist bumps into this closed space and measures the relative proportions of potassium-40 and argon-40. From this fraction, regardless of absolute quantities, knowing the half-life of potassium-40 and assuming that there was no argon at first, one can estimate the time elapsed since the start of the process, that is, since the clock "was reset." Notice that we need to know the ratio of the parent (potassium-40) and daughter (argon-40) isotopes. Moreover, as mentioned earlier, it is necessary that our watches be reset to zero.
But what is meant by "zeroing"? The crystallization process.
Like all radioactive clocks used by geologists, the potassium-argon time counting works only for so-called igneous rocks. Magmatic rocks harden from molten rocks - underground magma in the case of granite, lava from volcanoes in the case of basalt. When the rock solidifies, it crystallizes and forms granite or basalt. These, as a rule, small, transparent crystals, like quartz, are too small to look like crystals to the naked eye. Some of them, such as feldspars and mica, contain potassium atoms. Among them are the atoms of the radioactive isotope potassium-40. When a crystal forms at the time of magma solidification (the system “ closes ”), potassium-40 is present, but no argon (it is assumed that the bubbles of this gas, if any, have risen to the surface of the liquid lava and mixed with atmospheric air). The clock is “zeroed out” in the sense that there are no argon atoms in the crystal. After millions of years, potassium-40 slowly disintegrates, and, one by one, the argon-40 atoms replace the potassium-40 atoms in the crystal and remain in it, like a trap. The accumulated amount of argon-40 is a measure of the time elapsed since crystallization. But this value makes sense only when expressed as the ratio of potassium-40 to argon-40. When the clock was reset, the ratio was 100% in favor of potassium-40. After 1.26 billion years, the ratio will be 50 to 50. After another 1260 million years, half of the remaining 40 potassium will turn into argon-40, and so on. Intermediate proportions show intermediate times, since the time when the crystal clock was set to zero. Thus, by measuring the 40 K / 40 Ar ratio in a piece of igneous rock today, it can be said when the rock crystallized. Igneous rocks, as a rule, contain many different isotopes, and not only potassium-40. The positive point is that the igneous rocks in this piece harden at the same time, resetting all watches, which is very convenient for dating. However, during the crystallization of the mineral, argon may be trapped from outside. How to distinguish this argon from what was formed later during the decay of the 40 K isotope? It can be assumed that the captured argon had the same ratio of 40 Ar / 36 Ar isotopes, as in the modern atmosphere. By measuring the amount of 36 Ar, you can then calculate the amount of “pure” radiogenic argon 40 Ar.
However, there is a problem. Fossils are extremely rare in igneous rock. They form in sedimentary rocks such as limestone and sandstone, which are not frozen lava. They are found in layers of mud, silt or sand, which are gradually deposited on the bottom of the sea, lake or river. Sand or silt is compacted for centuries and hardens like stone. Remains trapped in sedimentary rock have a chance to be fossilized (to be preserved as a fossil). Although only a small part of the corpses becomes fossil, sedimentary rocks are the only ones that contain fossils that are worth talking about.
Unfortunately, these rocks cannot be dated by radioactivity. Probably, the individual particles of silt or sand that make up sedimentary rocks contain 40 K and other radioactive isotopes, but unfortunately, these clocks are useless because they are not zeroed properly, or zeroed at different times. Each grain of sand has a clock zeroed at one time, probably long before the formation of these rocks and the burial of minerals that we are trying to date. So, in terms of timekeeping, sedimentary rock is a mess. The best we can do, and this is a pretty good “best”, is to use the age of volcanic rocks that are close to or embedded in sedimentary rocks.
Fossil fossil dating is not required to literally find it pressed between two igneous rock plates, although this is a great way to illustrate the principle. In fact, a more sophisticated method is used. Recognizable layers of sediment are found throughout the world. Long before radioactive dating was discovered, these layers were identified and named: Cambrian, Ordovician, Devonian, Jurassic, Cretaceous, Eocene, Oligocene, Miocene. Devonian sediments are recognizable as Devonian, not only in Devon (a county in the south west of England, which gave them their name), but also in other regions. They are clearly similar to each other, and they contain the same types of fossils. Geologists have long known the order in which these deposits were deposited. Before the advent of radioactive hours, we simply did not know when they were formed. We could arrange them in order, because, obviously, more ancient sediments, as a rule, lie below the younger sediments. Devonian deposits, for example, are older than deposits of the Carboniferous period (so named because coal is often found in this layer), and we know this because in those parts of the world where these two layers meet in one place, the Devonian layer lies under the coal (exceptions are found in places where we can tell, based on other evidence, that the rocks were tilted, or even turned over). It rarely happens that there is a complete set of layers - from the Cambrian in the lower part to the modern at the top. But since the layers are so recognizable, one can determine their relative ages by building one after another and putting them together like a puzzle around the world.
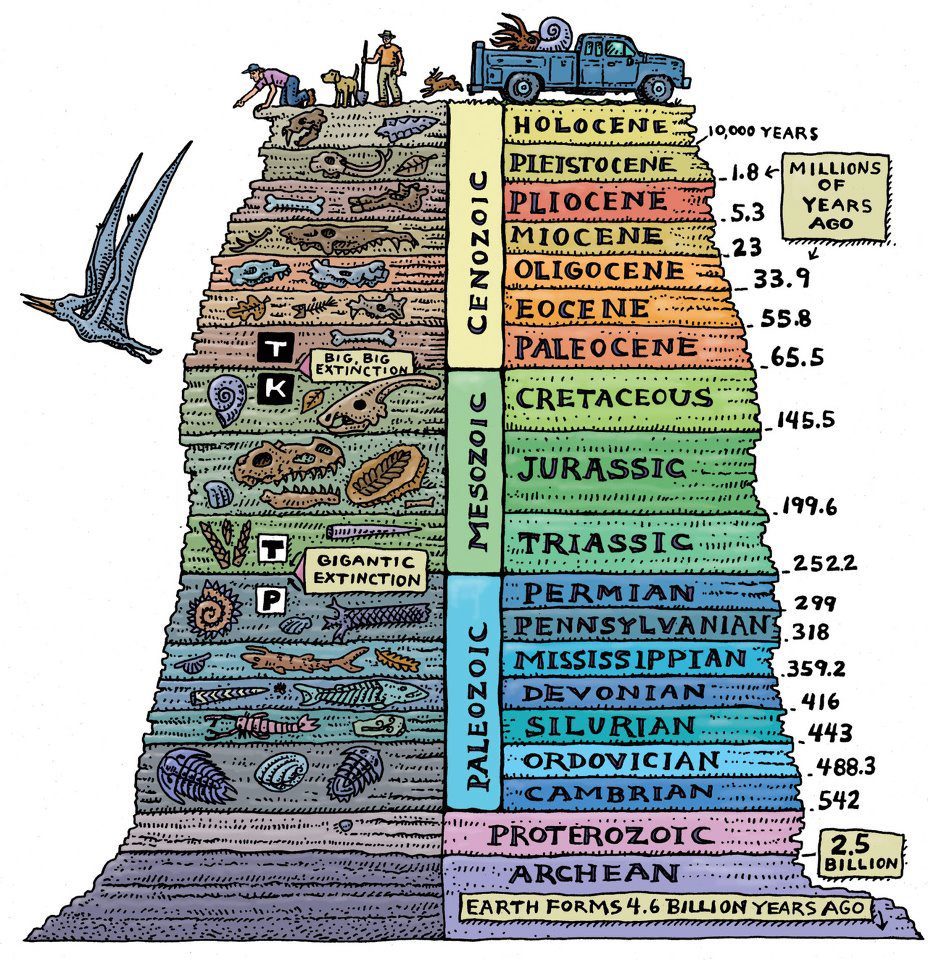
Let's return to dating. Since the relative order of the named sedimentary layers is well known, and the same order is found throughout the world, igneous rocks, which lie above or below the sedimentary layers, or that are embedded in them, can be used to date the named sedimentary layers, and therefore the fossils. inside of them. We do not need to look for igneous rocks in the vicinity of a particular fossil to date it. We can say that our fossils belong to, say, the end of the Devonian period, according to their position among the layers. And we know from the radioactive dating of igneous rocks discovered in connection with the Devonian layers throughout the world that the Devonian period ended about 360 million years ago.
The potassium-argon clock is only one of many clocks available to geologists who use the same principle on a different time scale. Faster clocks, such as carbon-14, work in a slightly different way for an interesting reason, namely, its reserves are constantly replenished. The role of carbon-14 in dating is somewhat different than that of more long-lived isotopes. In particular, what does it mean to “reset this watch”?
Carbon
Of all the chemical elements, this seems to be the most important for life, without which life on any planet is most difficult to imagine because of its remarkable ability to form chains, rings and other complex molecular structures. It is introduced into food chains with photosynthesis, a process in which green plants (and some bacteria and animals) absorb carbon dioxide molecules from the atmosphere and use the energy of sunlight to combine carbon atoms with water, creating sugar. All carbon in all living things comes, ultimately, through plants, from carbon dioxide in the atmosphere. And it comes back to the atmosphere when we exhale, when we excrete, and when we die.
Most of the carbon in the atmospheric carbon dioxide is carbon-12, which is not radioactive. However, about one atom per trillion is radioactive carbon-14. It decays rather quickly, with a half-life of 5,730 years, as already mentioned, into nitrogen-14. For plant biochemistry, there is no difference between these two isotopes. For plants, carbon is just carbon. Thus, plants include both of these kinds of carbon atoms in sugars in the same proportion as they are in the atmosphere.Carbon, which is part of the atmosphere (together with the same proportion of 14 C atoms ) quickly (compared to its half-life) spreads through the food chain, when plants are eaten by herbivores, herbivores by predators, and so on. All living things, whether plants or animals, have an approximately equal ratio of 14 C / 12 C, which is the same ratio as in the atmosphere.

So, when is this watch reset? At a time when a living creature, whether animal or plant, dies. At this moment, it is cut off from the food chain, and from the influx of fresh 14 C. Over the centuries, 14C in a corpse, or a piece of wood, or a piece of cloth, or other organic matter constantly decays into nitrogen-14. Therefore, the 14 C / 12 C ratio in the sample gradually falls below the standard ratio that living creatures share with the atmosphere. In the end, only 12 C will remain or, more precisely, the content of 14 C will be too small to measure. And the 14 C / 12 C ratio can be used to calculate the time that has passed since the date of death of the creature cut off from the food chain and its exchange with the atmosphere.

This is very good, but it only works because there is a continuous replenishment of 14 C in the atmosphere. Without this 14A C with a short half-life would have long disappeared from the face of the earth, along with all other natural short-lived isotopes. 14 C is special because it is continuously created by cosmic rays bombarding nitrogen atoms in the upper atmosphere.
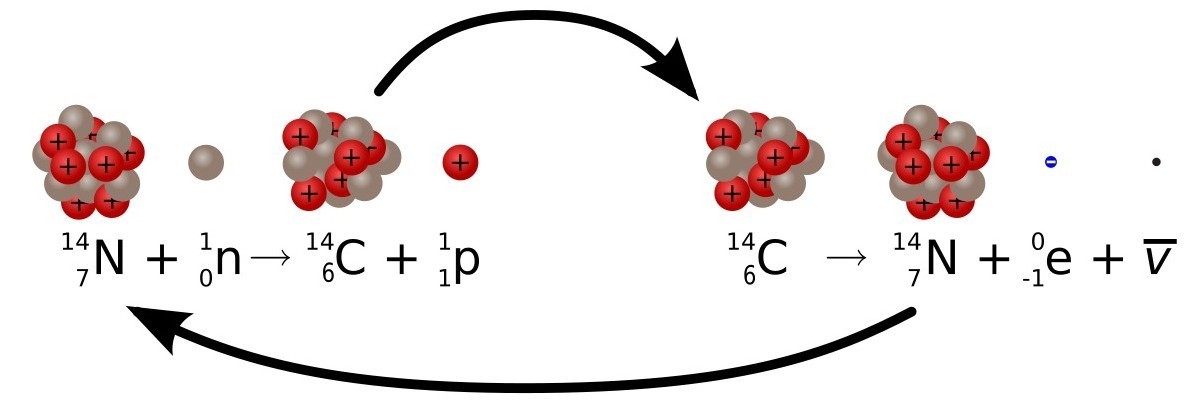
Nitrogen is the most common gas in the atmosphere, and its atomic number of 14 is the same as that of carbon-14. The only difference is that carbon has 14 protons and 8 neutrons, whereas nitrogen has 14 protons and 7 neutrons (neutrons, remember, have almost the same mass as protons). Particles of cosmic rays can, by bombarding a proton in the nucleus of a nitrogen atom, turn it into a neutron. When this happens, the atom becomes carbon-14, which stands one cell to the left of nitrogen in the periodic system. The speed of this transformation is approximately constant (depending on fluctuations of solar activity) and therefore radiocarbon dating works. Fortunately, we have accurate calibration of supply fluctuations 14C to the atmosphere, and we can amend them to refine our age calculations. Remember that, for about the same time range covered by carbon dating, there is an alternative method of dating wood - dendrochronology, which is absolutely accurate for up to a year. Looking at the radiocarbon-aged wood samples, whose age is independently set by dating using growth rings, we can calibrate this oscillating error in carbon dating. Now we can use these calibration measurements when we return to organic samples for which we do not have annual rings data (for most).
Radiocarbon dating is a relatively recent invention, it was proposed by Willard Libby.in 1946 (Nobel Prize in Chemistry, 1960). In the early years, substantial amounts of organic material were required for this procedure. Only in the 1970s, a technique called mass spectrometry was adapted for dating, and now only tiny amounts of organic matter are needed. This has revolutionized archaeological dating. The most famous example is the Shroud of Turin. Since this notorious piece of fabric was imprinted, it seems mysteriously the face of a bearded man (and, for unclear reason, for some reason in a cylindrical projection), many people hoped that it could occur from the time of Jesus. It first appears in the historical chronicle in the middle of the fourteenth century in France, and no one knows where it was before. She has been in Turin since 1578, and in the Vatican since 1983.When mass spectrometry made it possible to date on a tiny sample of the shroud, rather than a significant piece, which would have been necessary before, the Vatican made it possible to cut off a small strip. It was divided into three parts and sent to three leading laboratories specializing in radiocarbon dating, in Arizona, Oxford and Zurich. Working completely independently, without comparing records, these three laboratories submitted their reports on the date when the flax, from which the fabric is woven, died. The Arizona lab pointed to 1304, Oxford - to 1200, and Zurich - 1274 AD. All these dates are within the error, compatible with each other and with the date of 1350, in which the shroud is first mentioned in history. The dating of the shroud remains controversial, but not for reasons that cast doubt on the radiocarbon dating technique itself. For example,carbon in the shroud could be brought in by a fire that occurred in 1532. This is a good example to illustrate the method and the fact that, unlike dendrochronology, it does not have an accuracy of up to a year, only up to a century or so.
There are many different clocks that can be used, and they work best on different, but overlapping, time scales. Radioactive clocks can be used to independently assess the age of the same piece of rock, if you remember that all clocks were reset at the same time when this piece of rock crystallized. When such comparisons were made, different clocks were compared with each other - within the limits of the expected error limits. This gives greater confidence in the correctness of the clock. Thus, mutually calibrated and tested on known rocks, this clock can be applied with confidence to interesting dating problems, such as the age of the Earth itself. Currently established by Clare Patterson in 1956, an age of 4.55 ± 0.05 billion years is an estimate on which several different hours converge.
History of the establishment of the age of the Earth
[1946 .] (. . , ). , , . , , — . .
1948 . . — , , 1952 , — , .
, , , , . . . 1940- . , , , , . ( , , , .) , . : , . .
, , , , , . — ( ) .
, , . , - . , . , - , , . — , .
, , . 1953 . - — , , . , , , , .
— 4550 (- 70 ) — «, », . .
. , , , , , . : .
, , , , . . , , , , . , — - , — , . .
, — , , — . 90 % , , . . , 1923 , . , .
, , , (- ). , . , .
, 1923 , . . .
. «» . ( .) , , . , , , .
, . , 2000 The Nation, «» , « ». , , , , 1971 - .
, . 1970 « », 1986 . 80 %. - , , 625 , , . , , , - , . . « 44 », — . , , , 1993 .
« », , « », « » «» . ( 1962 « ».) , 2001 «» , « , ». — , , « ».
«» , , 2001 , 2000 25,1 ( 795 .), , 1999 (24,1 .), , 1998 (117 .). « , ». «» « .».
, , — , 1974 , , , (, ), , . , . , 27 . ? , , . 2010 .
1995 . . . , , , . , . ? . .
2001 Nature , .
, 1953 , .
1948 . . — , , 1952 , — , .
, , , , . . . 1940- . , , , , . ( , , , .) , . : , . .
, , , , , . — ( ) .
, , . , - . , . , - , , . — , .
, , . 1953 . - — , , . , , , , .
— 4550 (- 70 ) — «, », . .
. , , , , , . : .
, , , , . . , , , , . , — - , — , . .
, — , , — . 90 % , , . . , 1923 , . , .
, , , (- ). , . , .
, 1923 , . . .
. «» . ( .) , , . , , , .
, . , 2000 The Nation, «» , « ». , , , , 1971 - .
, . 1970 « », 1986 . 80 %. - , , 625 , , . , , , - , . . « 44 », — . , , , 1993 .
« », , « », « » «» . ( 1962 « ».) , 2001 «» , « , ». — , , « ».
«» , , 2001 , 2000 25,1 ( 795 .), , 1999 (24,1 .), , 1998 (117 .). « , ». «» « .».
, , — , 1974 , , , (, ), , . , . , 27 . ? , , . 2010 .
1995 . . . , , , . , . ? . .
2001 Nature , .
, 1953 , .
Criticism
So, the followers of the “alternative history” can declare, for example, that something is wrong with the potassium-argon clock. What if the modern very low decay rate of 40 K acted only after the Noah Flood? If before it the half-life of 40 K was radically different, and was, for example, several centuries, and not 1.26 billion years old? A special reservation in such a statement is striking. Why should the laws of physics change this way, Ad Hoc — so convenient and so large? It looks even more flashy if you need to make special mutually agreed reservations for each of the watches separately. Currently, all the methods used are consistent with each other in determining the date of the formation of the Earth in the range between four and five billion years ago. And they are based on the assumption that the half-life is always the same, which we fix today, as the well-known laws of physics directly prescribe them to be. History deniers would have to play around with the half-life of all isotopes in their different proportions so that they all agree with the assumption that the Earth was formed 6,000 years ago. This is what I call the special clause. Some other methods are not even mentioned here, for example, “track dating”, which also leads to the same result. It is necessary to take into account the huge differences in the time scales of different clocks, to think about the degree of tension and complexity of fitting the laws of physics, which would be necessary to make all clocks agree with each other in the range of several orders of magnitude, that the Earth is 6000 years old and not 4.55 billion ! Considering that the only motive for such adjustments is the desire to support the creation myth belonging to a private group of Bronze Age tribes, it is not surprising that mostly ignorant people are bought for it.
However, there are always mistakes. Buried organics can be contaminated with extraneous carbon, both "ancient" (with a low proportion of 14 C) and "young." As a result, there are, respectively, "aging errors" and "rejuvenation errors". In addition, the ratio of 14 C / 12 C in the atmosphere is not constant. For example, human activities and especially nuclear tests strongly affect this value. The formation rate of 14 C in the upper atmosphere depends on the intensity of cosmic and solar radiation, and these are variable values. The ratio of 14 C / 12 C depends on the total concentration of CO 2 in the atmosphere, the composition of which also varies. All these natural fluctuations, however, are not very large and they are taken into account. A really serious problem is only the possibility of sample contamination with foreign carbon. After all, the accuracy depends on the "people in the field" and on the laboratory. It is here that they are trying to compromise science, stating: “Scientists have determined the age of a living sheep is 15,000 years old!”, Silent about incorrect methodology - a sample could be taken from an animal grazing near the highway. And carbon got into the plants from car exhausts, which, burning refined petroleum products, release carbon from long-dead organisms.
As for the "Mesozoic hammer", "chains in coal", "trilobite crushed by a shoe" - when self-assessing the degree of reliability of such "news" you need to keep in mind that there should be a link to some article where it is described in detail - where, when, by whom and under what circumstances the find was made. Is it made by a scientist? There should be an archaeological context: a layer, which objects were nearby, and so on.
Information obtained through a chain of "testimony" - the most unreliable information (although it is taken somewhere in the courts). A typical example of such a distortion of information is a child's game "broken phone." I'm not talking about our imperfect perception and unreliable memory.
Check your watch
The inaccuracy of most methods of absolute geochronology does not give grounds to deny the accuracy of dating in archeology, paleontology and evolutionary biology (as do, for example, supporters of creationism, Fomenko's New Chronology and other pseudoscientific concepts). The main advantage of these methods is that there are many of them. And in the overwhelming majority of cases, they still give similar results, which, moreover, remarkably agree with the data on relative geochronology (the order of geological layers). If it were not so, there would be nothing to talk about! It is like with ship's chronometers: if he is alone, it is impossible to determine when he is lying; if there are two of them - it is already possible to understand that one of them is lying, it is unclear which of the two, and if there are three or more, the exact time can be known almost always.
For this reason, in scientific research, the age of objects is today decided to be determined using several independent methods. If this rule is violated, the result looks controversial for most professionals.
Finally, I apologize for a few "captain" style of presentation - it turned out that
Literature
1. Richard DOKINZ " The most ambitious show on Earth "
2. Site " Elements "
3. Bill BRYSON " A Brief History of Almost Everything "
4. Wikipedia
Source: https://habr.com/ru/post/390021/
All Articles