Liquid metal: pitfalls. Looking through the eyes of a chemist

Write this article I was inspired by the post NotSlow Liquid metal is not so terrible . Everything is simple there: he was insured against the circuit, applied a thin layer, fastened and rejoice at low temperatures. But is everything really good?
First you need to find out what kind of liquid metal it is. Among pure metals, the only one that can be liquid at room temperature is mercury. In their right mind, no one will now use mercury as a thermal interface due to its extreme toxicity and evaporation. The other two become liquid already at the temperature of the human body - it is cesium and gallium. Cesium is “fluorine reverse” in its chemical activity, it ignites and explodes from the slightest trace of air and moisture, and even destroys glass. Remains gallium (at KPDV it is him). At room temperature, gallium is still solid, but with some other low-melting metals, it forms eutectics, melting at 20.5 ° C (gallium-tin) and even 15.3 ° C (gallium-indium). Even lower — around 5 ° C — the gallium-indium-tin triple eutectic melts (62, 25, and 13%, respectively). Commercially available "liquid metal" type thermal interfaces are just alloys based on these three elements, possibly with some additional additives.
On this basis, the pitfalls are clear. The first of these is the absolute incompatibility of gallium-containing alloys with aluminum !
')
At a time when chemistry lessons at school were necessarily accompanied by a demonstration of experiments, experience in amalgamation of aluminum was among them. Aluminum was covered with a layer of mercury and he immediately began to rapidly oxidize, scattering right before his eyes. Mercury protected aluminum from the formation of an oxide layer and it was already formed on the amalgam surface, but it was not able to stop oxidation, since it was not kept on the surface of the liquid in a continuous layer, cracked, and a fresh, non-oxidized amalgam surface opened in the cracks.
The gallium alloy acts in exactly the same way, with the only difference being that it is capable of literally impregnating aluminum through, penetrating into intergranular gaps. Aluminum, saturated with liquid gallium, is not only oxidized before our eyes, but also crumbles in the hands.
So ZhM should be kept away from aluminum. And this applies not only to aluminum radiators: a random drop of "liquid metal" can destroy the body of the laptop, if it is from an aluminum alloy, and any other aluminum part. At least the case of some capacitor. Moreover, this drop is a classic catalyst - it does its dirty deed, without spending itself.
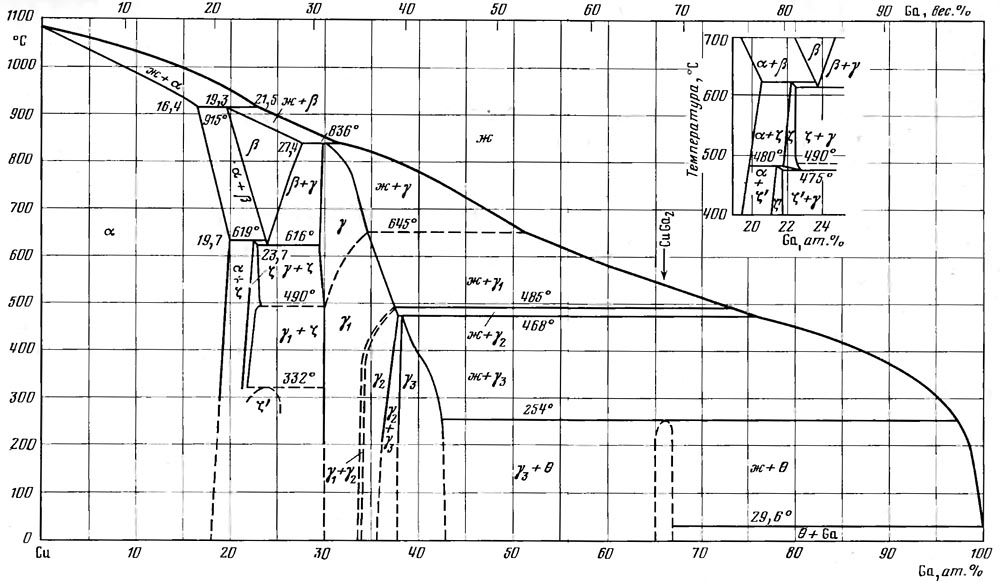
But also copper to gallium is not indifferent. In the figure above, I gave the Tx diagram of the copper-gallium system (from the reference book “State Diagrams of Binary Metallic Systems” edited by Lyakishev), which shows countless intermetallic compounds. As soon as gallium comes into contact with copper, they immediately begin to form. Liquid gallium (this also applies to its alloys) generally very readily wets both metals and non-metals, and the apparent chemical affinity greatly contributes to this. So the “liquid metal” will simply be absorbed into copper, forming an intermetallic crust on the border between the metals. The latter are not metals from the physical point of view, they are refractory, fragile and have poor thermal and electrical conductivity, but the main thing is that the “liquid metal” will be spent on their formation and will simply leave the gap. Many of those who have tried in the LM business report that over time it stops working, and after removing the radiator, they found that the liquid metal had "evaporated". He could not evaporate - a noticeable vapor pressure at his components appears only over a thousand degrees - he just soaked into copper, reacted with it. Nickel plating on copper helps to eliminate this phenomenon, although it is an additional obstacle to heat.
By the way, the absorption of gallium and its alloys into metals still applies to soldered joints - remember that little droplet that can destroy the aluminum case? So, the same droplet, which got on the solder, will make it brittle and solder unreliable. At some point it will work. Therefore, personally, I would keep the "liquid metal" as far as possible from any electronics.
And the last thing to write about: “liquid metal”, unfortunately, is harmless. According to some data, gallium is comparable in toxicity to arsenic, its second component, indium, is also a toxic heavy metal. Unlike mercury, gallium-based alloys are still absolutely non-volatile at ordinary temperature, so you cannot poison them with pairs, but because of their ability to easily stick to everything in the world, these alloys are incredibly mazuchy. To soil them, for example, hands is easier than light ones, and it is very difficult to wash them to the end. Then it all gets in your mouth. Therefore, we work with “liquid metal” and everything that contacted it only in rubber gloves and apart from food, drink and smoking . And yes, never do it like at KDPV!
Source: https://habr.com/ru/post/374331/
All Articles