Ask Ethan: Can ordinary stars synthesize elements heavier than iron?

In the cluster of Terzan 5 there are many old stars of low mass (dim red), but there are also hotter, younger stars of large mass, some of which can create iron and even heavier elements.
In the periodic table there are more than 90 elements that are naturally found in nature, but of all of them iron is the most stable. Synthesizing from lighter elements more heavy, and gradually approaching the gland, you get energy; the same thing happens if you split the heavier elements. Iron is the most stable configuration of protons and neutrons among all yet open atomic nuclei. And although this is only the 26th element, it represents the final stage of most of the fusion reactions even in the largest stars. But is it? This is what the reader is asking us:
Iron is called star fusion ash, which accumulates inside the stars, because it is the last element obtained as a result of the synthesis, which does not consume energy more than the synthesis does. I read about the r-process and other similar ones that lead to the appearance of heavier elements in new and supernovae stars. My next question is whether elements appear heavier than iron in ordinary stars, despite the fact that such a process absorbs more energy than it produces.
As you might have guessed, the answer to this question is rather complicated; elements in ordinary stars appear heavier than iron, but a very small fraction of them appears there as a result of synthesis.

Young star cluster in the region of formation, consisting of stars of very diverse masses. Some of them will burn silicon in their time, and in the process it will produce iron and many other elements.
All stars begin with the synthesis of helium from hydrogen - from tiny red dwarfs with a mass as small as 8% of the Sun’s mass to the largest, most massive stars of the Universe, with a mass of hundreds of suns. For 75% of all these stars, helium is the result of the reactions going on in them, but the more massive stars (and our Sun) go into the red giant phase, in which they will synthesize carbon from helium. But a very small percentage of stars - just over 0.1% - turn out to be the most massive, and are able to launch carbon-based synthesis, and further down the list. Only these stars will reach the supernova state, synthesizing oxygen from carbon, sulfur and silicon from oxygen, and then entering the final phase of silicon combustion before being transformed into a supernova.
')
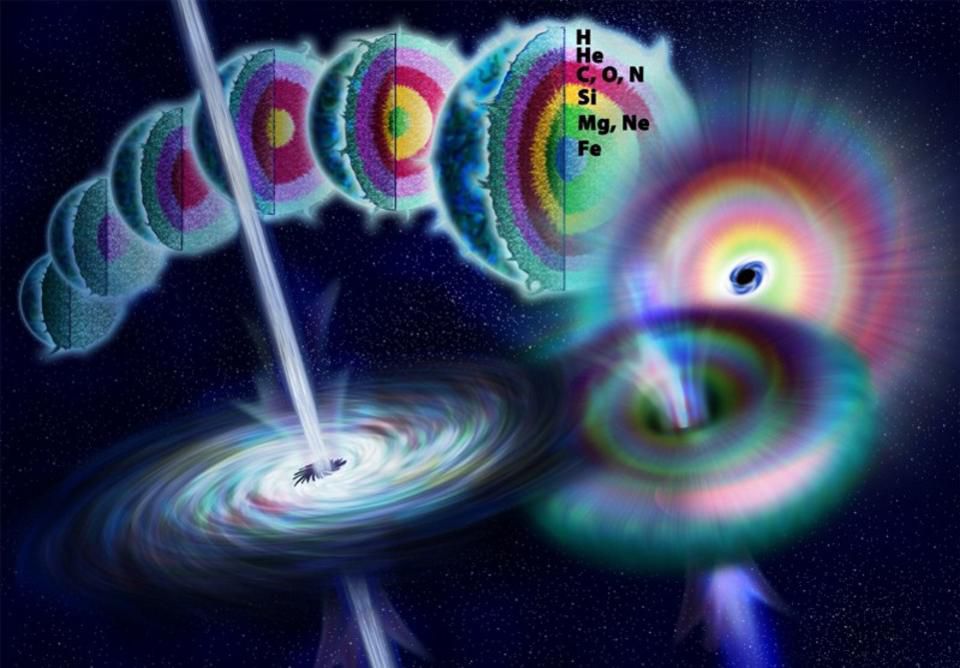
The anatomy of a very massive star during its lifetime, coming to a climax in the form of a type II supernova, when nuclear fuel runs out in the core. The final phase of the synthesis is the burning of carbon, as a result of which iron and iron-like elements appear in the core for a short time, and then a supernova explosion occurs.
This is the normal life cycle of the most massive stars of the Universe, but the burning of silicon is not like pushing two silicon nuclei together and the appearance of something heavier. Instead, a simple chain reaction occurs with the addition of a helium nucleus to the silicon core at temperatures exceeding 3,000,000,000 K, that is, more than 200 times the temperature in the core of the Sun. The chain reaction occurs as follows:
- Silicon-28 and helium-4 give sulfur-32,
- Sulfur 32 and helium-4 give argon-36,
- Argon-36 and helium-4 give calcium-40,
- Calcium-40 and helium-4 give titanium-44,
- Titanium-44 and helium-4 give chrome-48,
- Chromium-48 and helium-4 give iron-52,
- Iron-52 and helium-4 give nickel-56, and
- Nickel-56 and helium-4 give zinc-60.
Please note that iron-56 does not appear here, and for two reasons.
The iron and iron-like elements surrounding it are mainly obtained in the last moments of the life of an ultramassive star, shortly before the supernova explosion, in the process that occurs during the combustion of silicon.
One of them is clear from the periodic table - there are too few neutrons in the indicated part of it in the nuclei of the elements for the number of protons they have. For example, iron-52 is unstable; it emits a positron and decays to manganese-52, moving down the table (then manganese emits another positron and decays to a stable chromium-52). Nickel-56 is also unstable and decays to cobalt-56, which disintegrates to iron-56 - this is how we come to the most stable element of the periodic table. Zinc-60 decomposes first to copper-60, which decays to nickel-60. All of these end products are stable, so these stars — even before the supernova happens — can produce cobalt, nickel, copper, and zinc, and all this is heavier than iron.
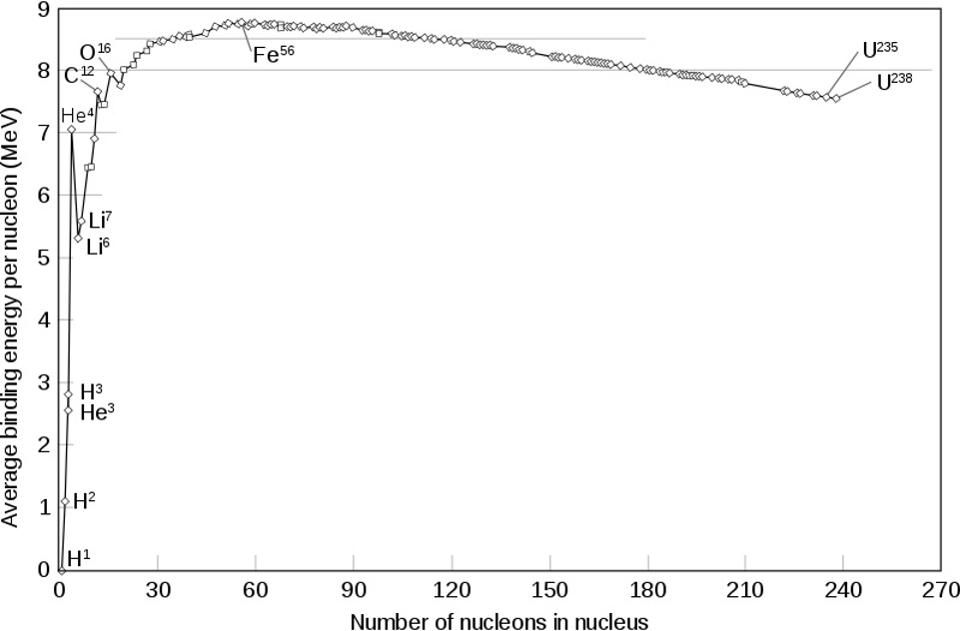
Iron-56 may be the most densely packed core, with the largest amount of binding energy per nucleon. However, slightly more or less heavy elements are almost as stable and strongly related, and the differences between them in this sense are very small.
But if this is not preferable energetically, how is this possible? Look at the graph just above, which shows the binding energy in terms of nucleon for each of the atomic nuclei. Note how flat the graph becomes in the area of iron-56; for many elements on both sides, the binding energy is almost the same. Now look left, find there Helium-4. What do you see?
Helium-4 is not as strongly bound as any nucleus in the region of iron-56. Therefore, despite, for example, the fact that zinc-60 has a lower binding energy per nucleon than nickel-56, it still has more binding energy per nucleon than nickel-56, together with helium-4. And in total, the reaction is obtained in a positive way. As a result, in the last moments of a star's life, before a supernova, it contains a mixture of all the elements up to zinc: four positions heavier than iron.
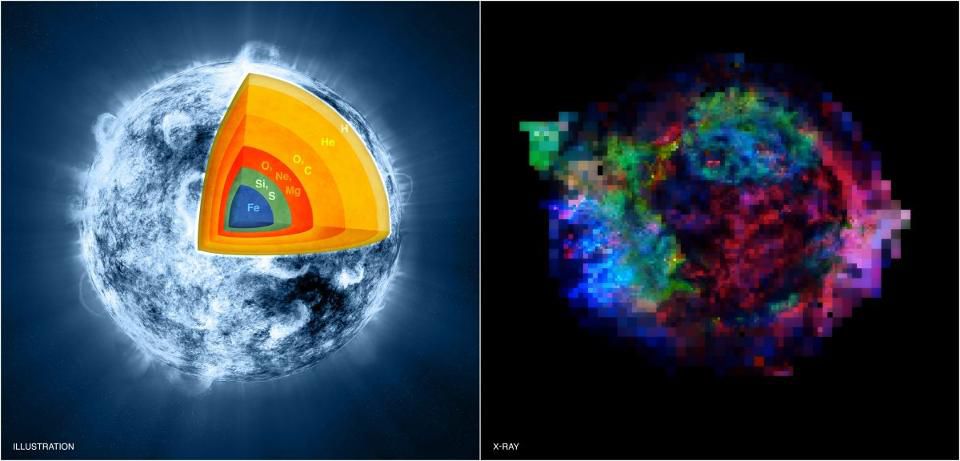
On the left, an illustration of the viscera of a massive star in the last stages of its life, before a supernova, during silicon combustion. On the right is an image from the Chandra telescope, where you can see the remnants of the supernova Cassiopeia A , and elements such as iron (blue), sulfur (green) and magnesium (red) are present.
Then you can ask about heavier items. Is it possible, for example, to add another helium-4 nucleus to zinc-60, and get germanium-64? In some residuals, probably, but not in large volumes. The thing is that the difference in energy is almost zero. And more importantly, your time is running out. For an extremely massive star, the duration of its various life stages is obtained as follows:
- Hydrogen synthesis: millions of years
- Helium synthesis: hundreds of thousands of years
- Synthesis of carbon: from hundreds to thousands of years
- Synthesis from oxygen: from months to one year,
- Synthesis of silicon: from several hours to one or two days.
In other words, the last stage, in which iron and iron-like elements appear, does not take too long for the process to go any further.
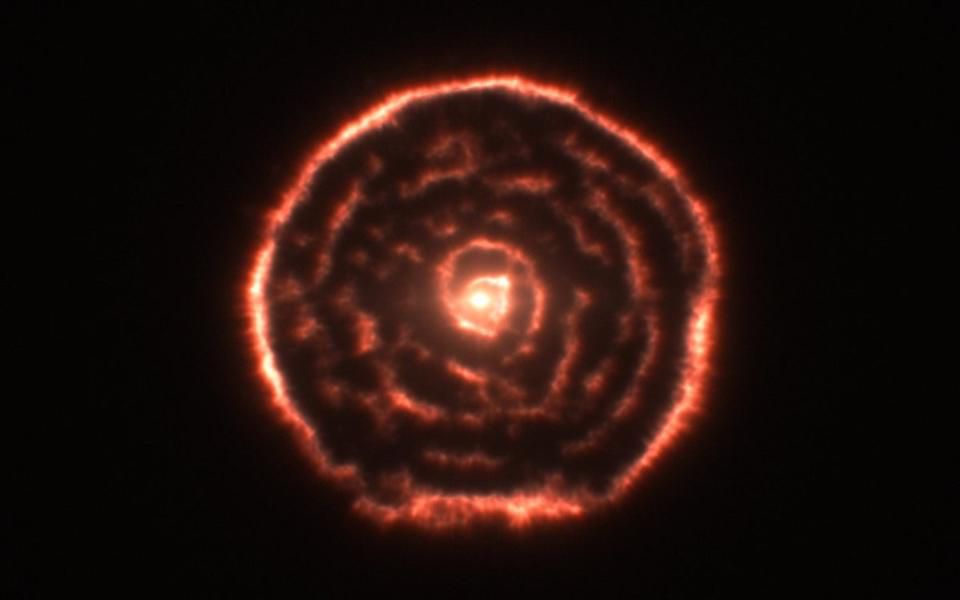
The spiral structure around the old giant star R Sculptor was formed due to the winds blowing the outer layers of the star during the AVG phase , where a huge amount of neutrons arise (from the synthesis of carbon-13 with helium-4).
But if you want to consider the processes occurring inside a massive star, in which iron and iron-like elements have already appeared, then you will be able to go all the way down to lead and bismuth. You see, after supernovae exploded in the universe, we get a lot of iron, cobalt, nickel, etc. - and these heavy elements are inside the new generations of emerging stars. In stars weighing from 60 to 1000% of the sun (usually not massive enough to become supernovae), synthesis can occur from carbon-13 and helium-4, and as a result oxygen-16 and a free neutron appear, and stars that will reach the supernova stage, will synthesize magnesium-25 and a free neutron from neon-22 and helium-4. In both of these processes, more and more heavy elements may appear, even lead, bismuth, and even (temporarily) polonium.
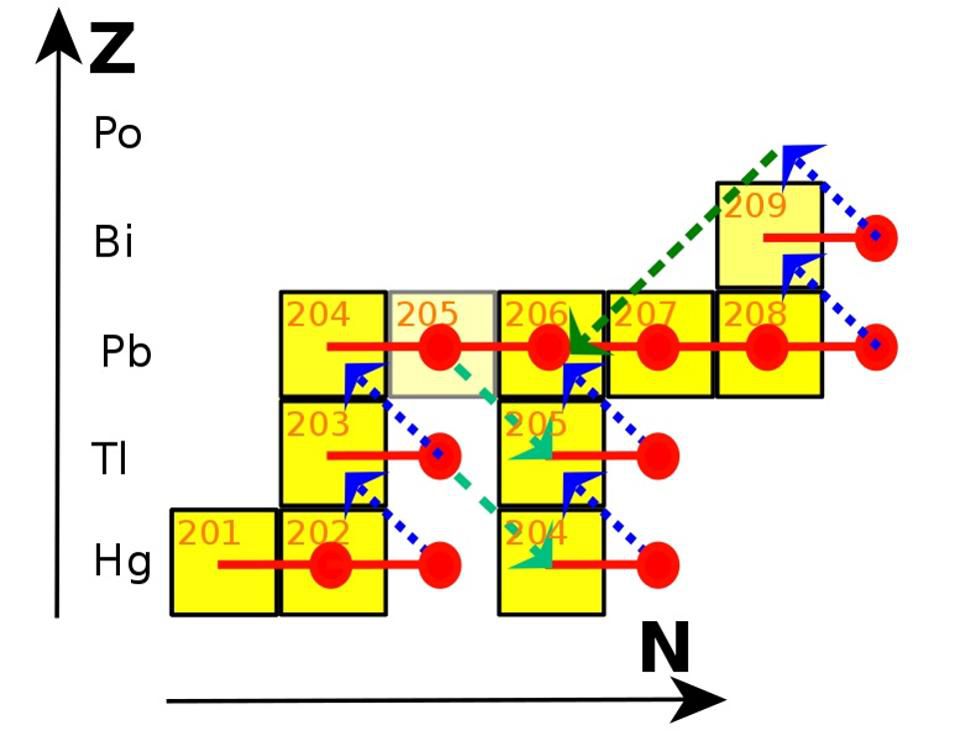
The graph of the last stage of the s-process (slow neutron capture). The red horizontal lines with a circle on the right are neutron capture; blue arrows pointing to the left and up - beta decay; green arrows pointing left and down - alpha decay; light green arrows pointing to the right and down - capture of electrons.
It may be ironic that it is the stars of great mass that produce a large number of light elements (up to rubidium and strontium - elements Nos. 37 and 38), and stars of low mass (not turning into supernovae) will take you to the end of this path, to lead and bismuth. Technically, it will not be a nuclear fusion reaction — it will be a neutron capture, but this is how more and more heavy elements appear. Metaphorically speaking, the main reason why low mass stars can reach such high altitudes is time.
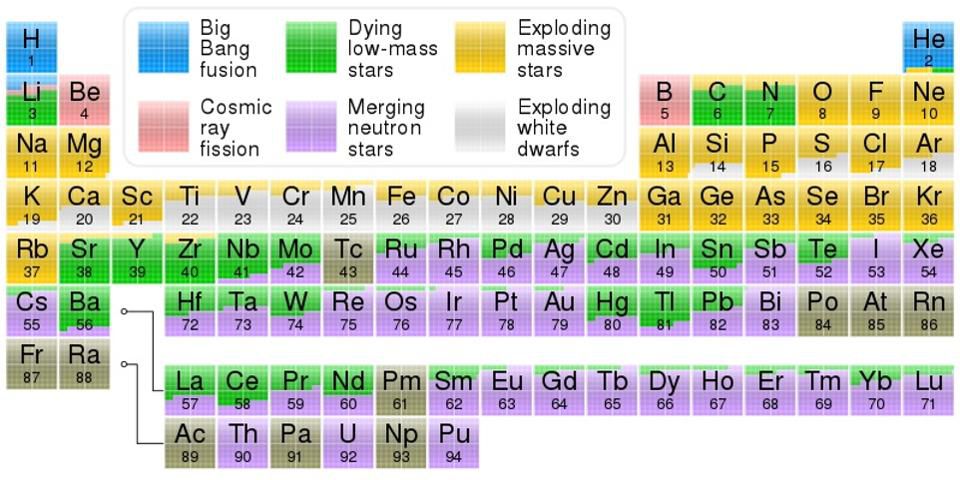
Periodic table showing the origin of elements in the solar system:
- Blue - synthesis during the Big Bang,
- Green - dying stars of low mass,
- Yellow - explosions of massive stars,
- Pink - splitting due to cosmic rays,
- Violet - neutron star fusion,
- White - white dwarf explosions.
Low mass stars remain in this state of neutron production for tens and even hundreds of thousands of years, and stars destined to become supernovae produce neutrons for only a hundred years, if not less. Energy issues play a very important role in the synthesis; even at temperatures of billions of degrees, reactions take place in an energetically preferable direction. But precious time is the most severe constraint for building increasingly heavier elements. Incredibly, with the right combination of neutron capture and nuclear fusion, almost half of all elements after iron can be obtained in stars, without any supernovae or a combination of neutron stars.
Ethan Siegel - astrophysicist, popularizer of science, blog Starts With A Bang! He wrote the books Beyond The Galaxy , and Treknologiya: Star Trek Science [ Treknology ].
FAQ: if the universe is expanding, why aren't we expanding ; why the age of the universe does not coincide with the radius of the observed part of it .
Source: https://habr.com/ru/post/374275/
All Articles