Chemists have found a way to effectively desalinate water and extract lithium from brine using MOF membranes, as in living cells.
A deficiency of lithium is one of the main reasons for the high cost of lithium-ion batteries, and as a result, the high cost of electric cars, where batteries account for more than half the cost. If it were not for the complexity of its production, then we would live in a completely different world, where alternative energy might have been much more widespread, and people would gradually forget about burning hydrocarbons in transport. But the reality is that there are not so many rich deposits: they are located in Argentina, Bolivia, Chile, China, the USA, Russia and several other countries.
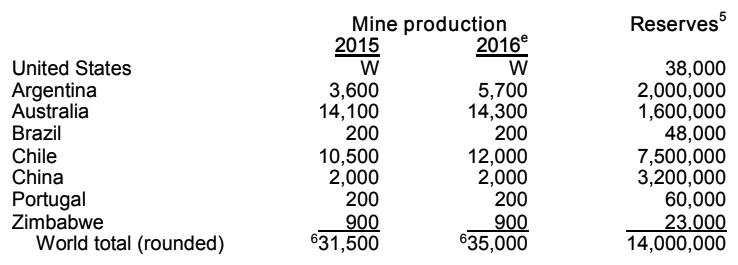
World reserves of lithium are large in themselves. Only in Russia there is 1 million tons of lithium, but here, after the collapse of the USSR, it is no longer mined: domestic enterprises import lithium-containing concentrate from Congo and China . The extraction of lithium carbonate Li 2 CO 3 involves the evaporation of brine in high salt lakes (Chile, Bolivia, Argentina, USA) or acid processing, as is the case with Russian ore. After evaporation, the carbonate is chlorinated to produce LiCl, electrolysis ( ) and vacuum distillation. All in all, around 35,000 tons of lithium are produced per year in world deposits.
Fortunately, an increasing percentage of lithium-ion batteries are being recycled, so now recycling yields a significant percentage of all lithium that goes to manufacturing facilities.
')
But lithium is still not enough. The demand for lithium constantly exceeds supply - and it grows every year. Perhaps this problem will be solved in an extraordinary way. The fact is that the huge reserves of lithium are contained in the World Ocean (huge reserves of gold and other metals are also dissolved in it). The question is how to get these riches cheaply and effectively. Chemists from the University of Texas at Austin (USA), Monash University, and the State Association of Scientific and Applied Research (both from Australia) have proposed a new efficient way to extract lithium and other metals from seawater.
They proposed a technological process using metal-organic frame membranes (MOF), which copy the filtration mechanism — ion selectivity — of biological cell membranes in living organisms. This highly effective easily separates metal ions. For his discovery in living cells, he was awarded the 2003 Nobel Prize in Chemistry. It can be used not only for processing sea water, but also for mountain ores.
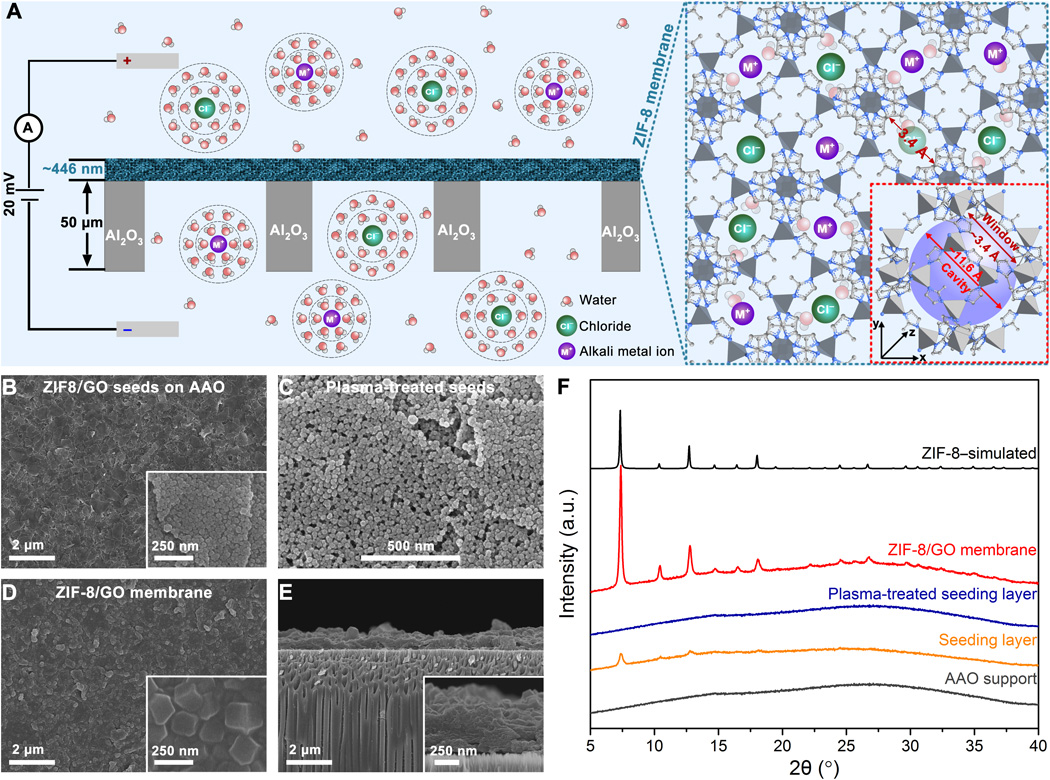
Schematic illustration of ion transfer through the ZIF-8 / GO / AAO membrane
Another by-product after filtering metals in salty water is fresh water that is suitable for drinking. That is, the process at the same time produces valuable ore and fresh water. “The prospect of using MOF to sustainably filter water is incredibly interesting from a public point of view, while the best way to extract lithium ions to meet global demand will help create new industries,” said Anita Hill, a leading researcher at the State Association scientific and applied research.
Effective desalination is the main task that researchers have set themselves. "But this is only a small part of the potential potential of this phenomenon," said Huanting Wang, a professor at Monash University. - We will continue to explore the selectivity of these membranes to lithium ions for practical use. Lithium ions in abundant quantities are present in seawater, so the discovery may be of great importance for the mining industry, where inefficient chemical methods are used to extract lithium from rocks and brines. The global demand for lithium required for electronics and batteries is very high. These membranes make it very efficient to extract lithium ions from seawater. ”
So in the future, the World Ocean can become a rich and easily accessible lithium resource. Perhaps, besides lithium, the membranes will be taught to use for filtering the gold and other metals needed by the industry.
The scientific article was published on February 9, 2018 in the journal Science Advances (doi: 10.1126 / sciadv.aaq0066).

World reserves of lithium are large in themselves. Only in Russia there is 1 million tons of lithium, but here, after the collapse of the USSR, it is no longer mined: domestic enterprises import lithium-containing concentrate from Congo and China . The extraction of lithium carbonate Li 2 CO 3 involves the evaporation of brine in high salt lakes (Chile, Bolivia, Argentina, USA) or acid processing, as is the case with Russian ore. After evaporation, the carbonate is chlorinated to produce LiCl, electrolysis ( ) and vacuum distillation. All in all, around 35,000 tons of lithium are produced per year in world deposits.
Fortunately, an increasing percentage of lithium-ion batteries are being recycled, so now recycling yields a significant percentage of all lithium that goes to manufacturing facilities.
')
But lithium is still not enough. The demand for lithium constantly exceeds supply - and it grows every year. Perhaps this problem will be solved in an extraordinary way. The fact is that the huge reserves of lithium are contained in the World Ocean (huge reserves of gold and other metals are also dissolved in it). The question is how to get these riches cheaply and effectively. Chemists from the University of Texas at Austin (USA), Monash University, and the State Association of Scientific and Applied Research (both from Australia) have proposed a new efficient way to extract lithium and other metals from seawater.
They proposed a technological process using metal-organic frame membranes (MOF), which copy the filtration mechanism — ion selectivity — of biological cell membranes in living organisms. This highly effective easily separates metal ions. For his discovery in living cells, he was awarded the 2003 Nobel Prize in Chemistry. It can be used not only for processing sea water, but also for mountain ores.

Schematic illustration of ion transfer through the ZIF-8 / GO / AAO membrane
Another by-product after filtering metals in salty water is fresh water that is suitable for drinking. That is, the process at the same time produces valuable ore and fresh water. “The prospect of using MOF to sustainably filter water is incredibly interesting from a public point of view, while the best way to extract lithium ions to meet global demand will help create new industries,” said Anita Hill, a leading researcher at the State Association scientific and applied research.
Effective desalination is the main task that researchers have set themselves. "But this is only a small part of the potential potential of this phenomenon," said Huanting Wang, a professor at Monash University. - We will continue to explore the selectivity of these membranes to lithium ions for practical use. Lithium ions in abundant quantities are present in seawater, so the discovery may be of great importance for the mining industry, where inefficient chemical methods are used to extract lithium from rocks and brines. The global demand for lithium required for electronics and batteries is very high. These membranes make it very efficient to extract lithium ions from seawater. ”
So in the future, the World Ocean can become a rich and easily accessible lithium resource. Perhaps, besides lithium, the membranes will be taught to use for filtering the gold and other metals needed by the industry.
The scientific article was published on February 9, 2018 in the journal Science Advances (doi: 10.1126 / sciadv.aaq0066).
Source: https://habr.com/ru/post/374265/
All Articles