Kenneth Snelson Chemistry (Part I)
In justifying the properties of atoms and molecules, it is customary to refer to the postulates of quantum mechanics, which far from all physicists understand. Moreover, chemists whose principles of Pauli and Heisenberg, the rules of Klechkovsky and Hunda, and even the Schrödinger equation do not evoke any feelings, except a feeling of deep respect for the above-mentioned physicists. It is even worse for the humanities and other artistic artists who are useless to describe and explain such principles, rules and equations. As a result, one of them - artist Kenneth Snelson (Kenneth Snelson; 06/29/1927 - 12/22/2016) - decided that "the salvation of the drowning is the work of the drowning". And in 1960 he invented a simple theory of the structure of the atom, to which he dedicated several dozen of his paintings, and even carved out of granite / 1 /.
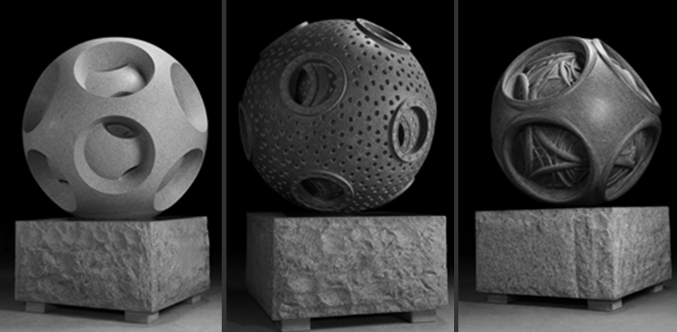
Fig. 1. “Atomic” sculptures (4'x4'x4 ', granite, 2009) / 1 /
Electrons in the atomic model of Snelson have a ring shape and form spherical electron shells consisting of contiguous electron rings (“circle-sphere”). Models of such "cyclospheres" Snelson built from ferrite ring magnets. If they are located on the surface of a sphere, then, when the direction of the magnetic field alternates, the edges of the adjacent magnets attract each other, and their outer planes form polyhedral (ring-bound) shells.
')
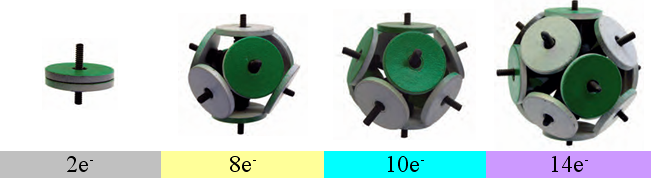
Fig.2. Magnetic models of Snelson electron shells
The most stable "electronic" structures are obtained from two, eight, ten and fourteen magnets.
According to Snelson's theory, such stable combinations of magnets correspond to the four main types of filled atomic shells constructed from ring electrons. The first type (2e - ) corresponds to helium, the atomic radius of which is 31 pm. The second (8e - ) is characteristic of noble gases, closing the periods in the periodic table (Ne - 38 pm; Ar - 71 pm; Kr - 88 pm; Xe - 108 pm; Rn - 120 pm). Larger filled shells (10e - ) have nickel subgroup elements (Ni - 149 pm; Pd - 169 pm; Pt - 177 pm). A shell of 14 electrons can form in lanthanides (Er - 226 pm) and, possibly, in actinides. In this case, the grouping of chemical elements according to the types of external electron shells of atoms reflects their division into small (8e - ) and large (10e - + 8e - or 14e - + 10e - + 8e - ) periods of the Periodic Table.
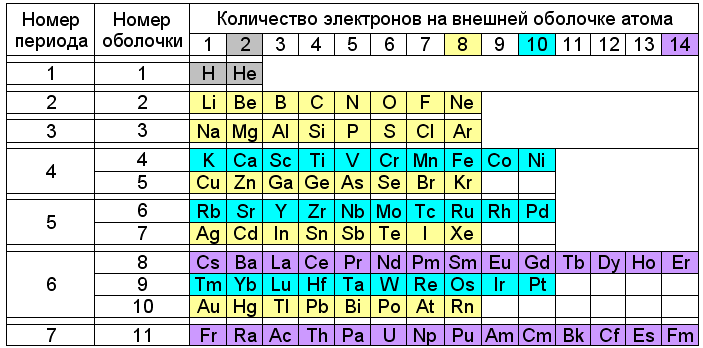
Fig. 3. Periodic table with division of periods according to Snelson
The position of some elements in this table looks strange. For example, sodium, rubidium and cesium, despite the similarity of chemical properties, turn out to be in different series of colors - yellow (8e - ), blue (10e - ) and lilac (14e - ). Copper, silver and gold are colored the same way as lithium and sodium are yellow. But keep in mind that the external electrons of elements from lithium to neon and from sodium to argon form eight-electron shells, and from potassium to nickel and from copper to krypton - ten-electron. On the same basis, elements from copper to krypton, from silver to xenon and from gold to radon are highlighted in yellow, and blue from rubidium to palladium and from thulium to platinum.
In this case, the chemical properties of elements with one electron on the outer shell are determined by the type of the underlying filled electron shell — eight-electron for alkali metals and ten-electron for copper, silver, and gold.
The radii of atoms in the periodic table change periodically — they increase spasmodically in the transition to each subsequent period, but within periods they decrease.
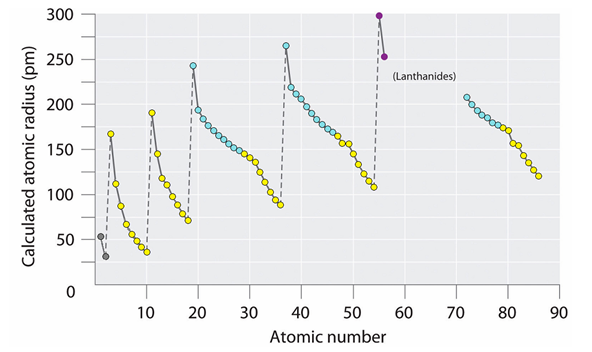
Fig. 4. Periods of atomic radii reference (with modifications)
From this it follows that the radii of ring electrons in atoms are also variable and depend on the distance to the atomic nucleus, its charge, and the type of electron shell. They are maximal on the outer shell, and are especially large at the beginning of the period when the charge of the atomic nucleus (for a given period) is minimal. In this case, the type of the filled electron shell depends on the atomic radius. If it is not more than 120 pm, then eight-electron shells are obtained. If more than 140 pm, then ten-electron. The construction of a filled shell for larger lanthanides (> 200 pm) requires no less than 14 electrons.
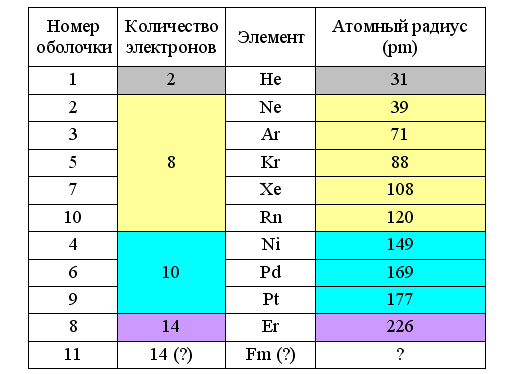
Fig. 5. Atomic radii with filled electron shells ( reference )
In the periodic table with periods separated by Snellson, the position of noble gases with completed eight-electron shells looks quite logical. It is logical to look at it and the elements with filled ten-electron shells. These are silvery metals characterized by high chemical resistance and plasticity (nickel, palladium and platinum). But the construction of the 14-electron shell of lanthanides should be completed in erbium, which does not differ in any way from the preceding dysprosium and holmium, or from the thulium, yttrium and lutetium that follow it. But on going from lutetium to hafnium, there is a sharp increase in density - from 9.85 to 13.2 g / cm3. Apparently, only at this stage, due to a decrease in the atomic radius, it becomes possible to form a stable 14-electron shell. And with its formation, there are "extra" external electrons. Therefore, in nature, there is no element with a 14-electronic complete outer shell that is noticeably different in chemical properties from neighboring elements.
The cyclosphere theory of the structure of the electron shells of atoms, proposed by Snelson, is in good agreement with the periods of the periodic table, so it would be interesting to evaluate its consistency with the properties of molecules.
The outer shell of eight electrons, characteristic of noble gases, may have even the simplest molecules containing hydrogen. For example, a methane molecule (CH 4 ). Usually in its models hydrogen is designated by a hemisphere or a separate ball. In the cyclospheric (ring-shaped) model, the eight-electron shell of methane consists of four ring electrons of the outer shell of a carbon atom and four hydrogen atoms (protonated electrons).
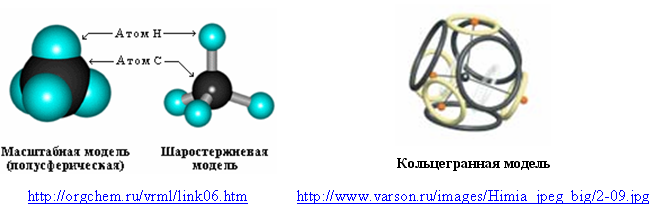
Fig. 6. Models of methane molecule
The attraction of “hydrogen” electrons to protons reduces their diameters, and the repulsion of protons from a positively charged nucleus slightly pushes them out of the plane of the electron rings. But if you make the electrons invisible and connect the carbon nucleus to the protons by lines, then the ring-grit model of the methane molecule will take on a classic appearance - symmetrical, with the same angles (109.5 °) between all hydrogen atoms.
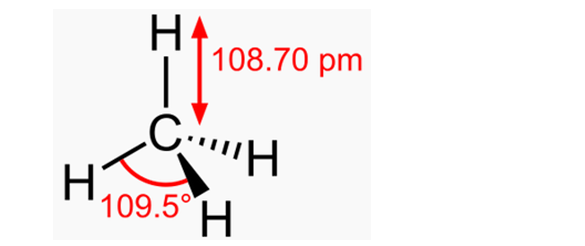
Fig. 7. The structure of the methane molecule
Ring-bound electron shells of the borohydride anion (BH 4 - ) and the ammonium cation (NH 4 + ) may look similar. But the borohydride should be slightly larger, and the ammonium should be slightly less methane.
The ammonia molecule (NH 3 ) also has an eight-electron shell, although the loss of one ammonium proton deprives it of a positive charge, breaks the symmetry and slightly changes the angles of location of the three remaining protons. And the same electron shell, but slightly smaller in diameter, should be near the hydroxonium cation (H 3 O + ).
By the same principle, it is possible to construct an eight-electron shell of a water molecule, in which the angle formed by the oxygen nucleus and two protons will be even smaller.
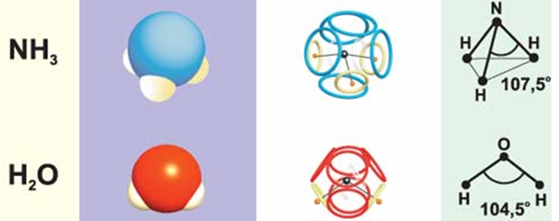
Fig. 8. Models of ammonia and water molecules / 2 / ( picture )
Thus, it is possible to identify a number of simplest molecules and ions with eight-electron shells, in which the shortage of external electrons is compensated by hydrogen atoms (protonated electrons). For the elements of the second period, these are BH 4 - , CH 4 , NH 4 + , NH 3 , H 3 O + , H 2 O, OH - , HF and F - . Neon closes the second period, which does not require protons to neutralize the total negative charge of electrons of the outer shell of an atom.
By the same principle, external eight-electron shells are constructed for SiH 4 , PH 3 , SH 2 and HCl molecules, formed by elements of the third period of the Periodic Table. The hydrogen atoms involved in the construction of the electron shells of such molecules and ions affect their shape and chemical properties, but do not change the overall structure.
In all such models, the interposition of heavy nuclei with an inner electron shell and “bare” protons is in good agreement with the well-known “canonized” structures of these simplest molecules. And according to Snelson's theory, the interposition of the central nucleus and protons is explained by the stability of the eight-electron outer shells of similar molecules formed by atoms of the second and third periods of the Periodic Table.
The similarities and differences in the electron shells of such simplest molecules well reflect (and explain well) their known structural parameters, but with water molecules everything can be more complicated. In addition to the usual "meta-water", in which the protons are located at an angle of 104.5 °, there must be a "steam-water" in which the protons lie on the same straight line with the nucleus of oxygen (180 °). Moreover, the "meta-water" nuclei of hydrogen atoms (protons) can be either in the "northern" (N) or in the "southern" (S) electrons. In "para-water" such magnetic isomerism is absent, since its proton-containing electrons have opposite (relative to the center of the molecule) orientation of the magnetic field.
The structural and magnetic isomerism of the electron shells of water molecules in cyclospheric models contradicts its generally accepted descriptions. This could serve as proof of the fallacy of Snelson's theory, if it were not for the surprising diversity of the properties of water, which is not explained within the framework of generally accepted ideas about the only variant of its molecular structure.
Magnetic isomerism should be characteristic of electron shells and many other molecules. But this topic, as well as the theoretical justification of the properties of water, it is better to discuss separately.

Fig. 9. Magnetic isomers of the simplest molecules (cringling models of electron shells)
Separate consideration is given to the numerous carbon compounds in which the electron shells should differ noticeably from the shells of the simplest molecules described above.
For compounds consisting only of carbon, the extreme diversity of molecular structures that form materials with unique properties (carbin, graphene, fullerene, nanotubes, diamond, etc. / 3 /) is characteristic. After awarding the Nobel Prizes to the discoverers of fullerene (1996; R. Curl, H. Kroto, R. Smolli) and graphene (2010; AK Geim, KS Novoselyov), predictions and searches for new forms became more popular carbon. As a result of such predictions, molecules of graphite, grafdin, phagrafen, and other predominantly hypothetical carbon compounds have been described.
The simplest arrangement is carbyne - a linear polymer of carbon / 4 /. Assembling a model of its electron shell is not difficult, since there are four electrons on the outer shell of a carbon atom. These electrons form a "cube" with two unfilled faces. The assembly of such "cubes" of the linear polymer gives carbin.
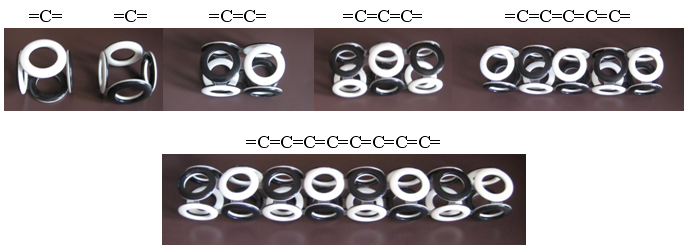
Fig. 10. Carbin
The high fragility of carbyne allows it to bend and form closed rings. Due to the obligatory alternation of the poles of the magnetic field in adjacent electrons of the ring, there can be only an even number of carbon atoms. Rings with six carbon atoms (hexamers, C 6 ), whose electrons can connect at different angles and form electron shells of various types, should be especially stable. The most famous structures form the "Nobel" molecules - graphene and fullerene. It is not difficult to construct for them models of electron shells of the Snelson type, if we use different types of hexamer carbon rings, such as graphene (2D) and fullerene (3D), in this construction.
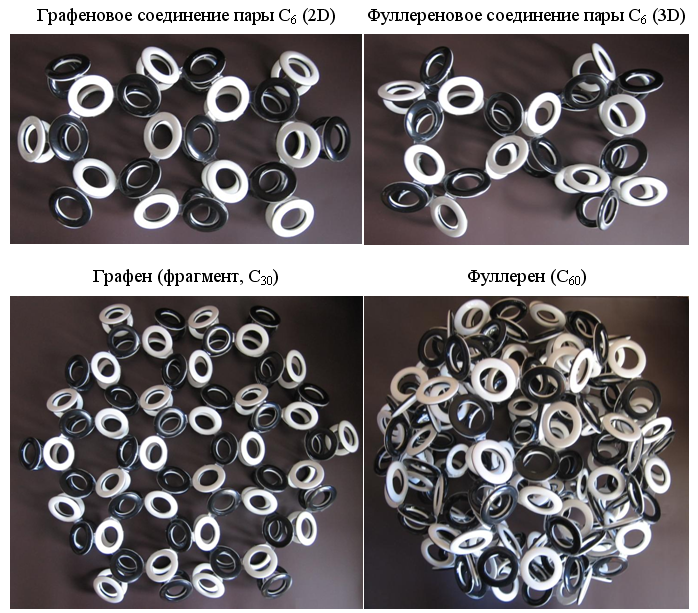
Fig. 11. Main 2D and 3D carbon forms
Fullerenes got their name in honor of the outstanding American architect, designer and inventor Richard Fuller. But a great contribution to the theory of the famous Fuller was made by his less famous employee and not less talented inventor Kenneth Snelson. Therefore, it would be nice to give his still undiscovered carbon molecules its name. Moreover, the Snelson theory allows not only to describe the already known forms of carbon, but also to predict its yet unknown varieties. Many such structures can be built, but, as a rule, they are distinguished not only by increased flexibility and fragility, but also the ability to form cohesive three-dimensional conglomerates, which makes it difficult to separate individual chemicals from a mixture of similar structural analogs.
It is also possible to build electron shells of the Snelson type for the most durable form of carbon - diamond. And not only for its usual variety, but also for a more rare and poorly studied form - lonsdaleite.

Fig. 12. The structure of the diamond electron shells
The diamond crystal lattice can be considered a three-dimensional polymer of methane, in which all hydrogen atoms are replaced by carbon-carbon "hole" bonds. Each carbon atom is surrounded by four electrons and four interatomic bonds, with small gaps between the electron shells of individual atoms. In addition, each electron is linked with six neighboring electrons.
In a lonsdaleite crystal, carbon atoms have eight-electron shells, and all the electrons forming them serve as interatomic septa (“electronic” bonds). As a result, for each carbon atom there are four “whole” electrons. More precisely - eight of their "halves", divided between adjacent atoms. Unlike ordinary diamond, lonsdaleite has no interatomic gaps. In addition, each electron is also associated with six neighboring electrons.
Similar variants of the structure of the electron shells can have crystals and other particularly hard substances — moissanite (SiC), borazone (γ-BN), and boron phosphide (BP). The high hardness of all such compounds is well explained by the structure of their electron shells, in which each electron is associated with six neighbors. In ordinary molecules, electrons have no more than four such bonds.
The Snelsonian theory of the structure of molecular electron shells rather well explains the diversity of allotropic forms of carbon, but to prove its universality, it is advisable to test this theory on other carbon compounds. First of all - on well-studied hydrocarbons.
Alkanes, i.e. saturated hydrocarbons with the general formula C n H 2n + 2 can be considered derivatives of methane, in which some proton electrons are replaced by “hole” carbon-carbon bonds. Three of the simplest molecules of this type include ethane, propane, butane and isobutane.

Fig. 13. Models of electronic shells of simplest alkanes
For propane and other alkanes having an odd number of carbon atoms in an unbranched chain, magnetic isomerism is characteristic. In linear saturated hydrocarbons with an even number of carbon atoms, magnetic isomerism is absent. And with an increase in the number of carbon atoms in a molecule, the number of magnetic, structural and rotational isomers (rotamers) increases exponentially.
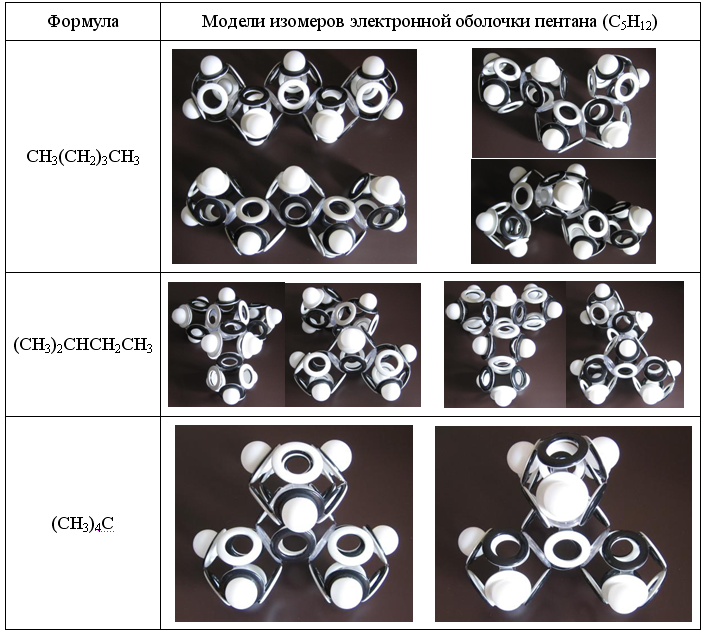
Fig. 14. Magnetic, structural and rotational isomers of pentane
Even more isomers can be constructed for hexane, but this does not apply to cyclic saturated hydrocarbons. For example, cyclohexane has no magnetic isomers, although if we look at the model of its electron shell from the side, we can see that the unidirectional spins of the upper and lower electrons have the opposite orientation.
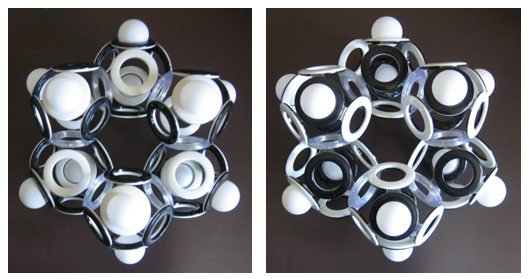
Fig. 15. Model of the electron shell of cyclohexane (C 6 H 12 ) (two sides of the same "medal")
The original electron shell has a benzene molecule (C 6 H 6 ) containing six symmetric planar hydrogen atoms. It is impossible to build such a shell from the hexamer C 6 , characteristic of graphene or fullerene. But it is easily built on the basis of a ring of six interlinked electrons, forming a central hexagonal cylinder. In this construction, for each carbon atom there are five electrons - four of its own and one "hydrogen". This structure explains both the low density of benzene (0.876 g / cm ³), the symmetry of the structure of the molecule, the planarity of the arrangement of hydrogen atoms, and their ability to be replaced by radicals, which lack one electron to complete the octahedral shells.

Fig. 16. Electron shell of a benzene molecule
Typical for benzene carbon atoms, the five-member compounds of ring electrons are repeatedly found in Snelson's paintings, and it would be strange if they were absent in chemical compounds.
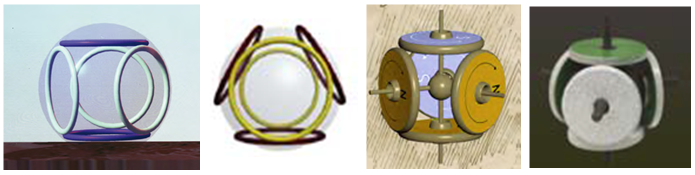
Fig. 17. Five-electron shells of Snelson / 1 /
Based on the above described version of the electron shell of benzene, it is possible to construct shells for its numerous derivatives with various side radicals, as well as with nitrogen atoms, partially or completely replacing the central ring carbon.
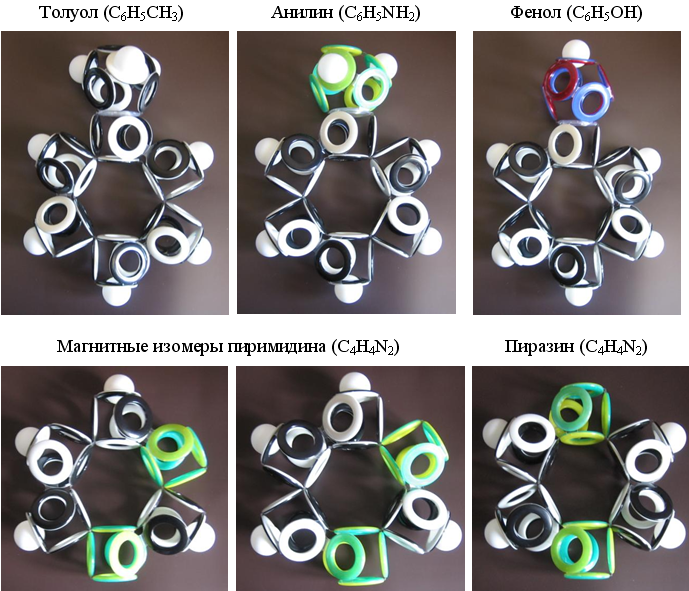
Fig. 18. Models of electron shells of simplest benzene derivatives
The outer electron shell of ethylene (C 2 H 4 ) —the simplest representative of alkenes (C n H 2n ) —is easily constructed from eight ordinary and four protonated electron rings. Analogous 12-electron shells can have molecules of diimide (N 2 H 2 ), oxygen (O 2 ) and formaldehyde (H 2 O).
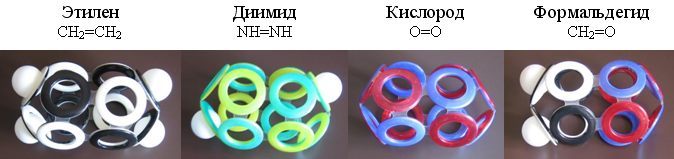
Fig. 19. Mock-ups of molecules with 12-electron shells.
All other alkenes, dienes and trienes can be considered alkanes containing one, two or three ethylene groups.
Hydrocarbons belonging to alkynes (C n H 2n-2 ) contain a pair of carbon atoms combined by an elongated 10-electron shell. For each of the carbon atoms there are four electrons; therefore, the missing positions of acetylene are built up with two hydrogen atoms, and for the remaining alkynes, these hydrogens are replaced by one or two “hole” bonds with the electron shells of neighboring atoms.
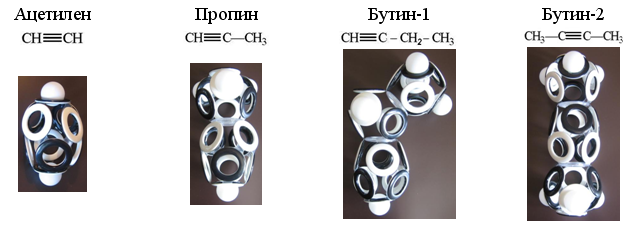
Fig. 20. Dummy shells of simplest alkynes
Thus, for all major types of hydrocarbons — alkanes, arenes, alkenes, and alkynes — models of external electron shells can be constructed that satisfy all the canons of Kenneth Snelson's theory. Moreover, such shells can serve as samples for molecular prototyping of compounds containing not only carbon atoms. Illustrative examples are the 12-electron shells of ethylene analogs - diimide, oxygen and formaldehyde described above. By analogy with them, it would be interesting to consider the Snelsonian shells of more complex molecules containing 10-electronic structures of acetylene type.
The spherical shell, built of ten electronic rings, is capable of stretching in different directions. Best of all - along the axis of symmetry passing through a pair of electrons at the "poles". In chemically unstable acetylene (C 2 H 2 ), this axis passes through hydrogen atoms. In the chemical resistance of molecular nitrogen (N 2 ), hydrogen atoms are absent, and the elongated electron shell is characterized by increased strength. A similar Snelson 10-electron shell can be constructed for some other simplest molecules.
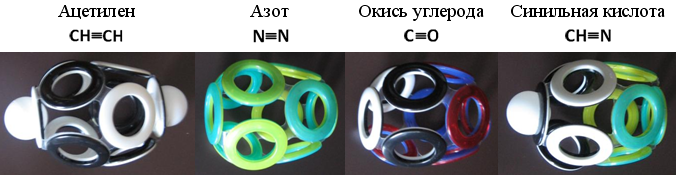
Fig. 21. Layouts of 10-electron shells of simplest molecules
More complex electronic shells containing inserts of 10-electronic elements may have molecules of urea, acetone, acetamide, carbonic and acetic acids, as well as guanidine. A characteristic feature of all molecules of this type is their magnetic isomerism and the presence of interatomic bonds with electronic inserts ("electronic" bonds).

Fig. 22. Layouts of molecular shells with 10-electron central groups.
Especially often in organic compounds occurs 10-electron group of carbon monoxide (CO). Some heterocyclic compounds contain two, three or even four such groups.
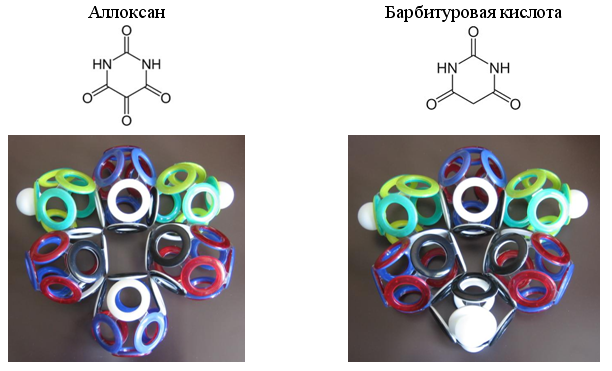
Fig. 23. Examples of heterocycles with 10-electron groups WITH
A characteristic feature of the electron shells of such molecules is the presence of interatomic bonds of the lonsdaleite type (with insertions of interatomic electrons). Similar "electronic" bonds may be present in many other molecules. For example, in polyiodides (I 3 - , I 5 - , I 7 - , I 9 - and others), which crystallize from solutions containing iodides (I - ) and iodine (I 2 ) / 5 /.

Fig. 24. Layout of the electron shell of anion I 3 - with interatomic bonds of lonsdailite type
It would be very interesting to analyze the structure of the electron shells of numerous halogen compounds, but only for a limited circle of chemists. Therefore, it is better to check the correctness of the theory proposed by Snelson by constructing the electron shells of more well-known molecules - proteins, DNA, etc ...
The main structural element of all protein molecules is a peptide bond. The coincidence of its known parameters with the parameters of a ring-shaped electron shell can serve as a good proof of the correctness of Snelson's theory. A mismatch - a refutation.
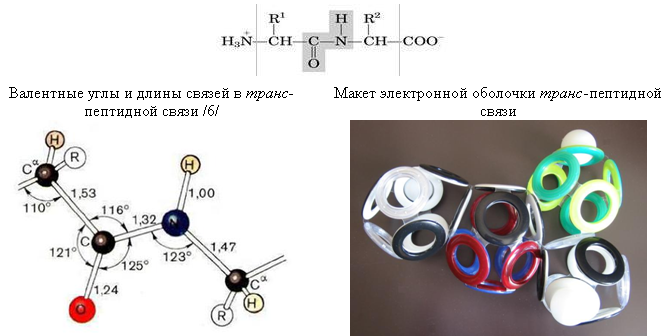
Fig. 25. Peptide Coupling
The cringing model displays only the electron shell, which does not allow to accurately determine the angles between the centers of the atoms. However, their general arrangement is consistent with the schemes from textbooks / 6 /. For example, the layout clearly shows the planarity of the three bonds of the peptide nitrogen atom, which in the majority of its compounds are located pyramidally. Such a planarity can be explained by the presence of an interatomic electron between the 10-electron shell of CO and the octahedral shell of the nitrogen atom.This electron is adjacent to the unfilled electron ("hole") bond NC (indicated by a transparent grommet), and the most stable position for the protonated electron (hydrogen atom) in this case becomes the opposite face of the shell of the nitrogen atom.
The decrease in the peptide bond length (CO) -N (1.32 Å) compared with the length of the single CN bond (1.47 Å) can also be explained by the presence of an interatomic ("lonsdailite") electron in it. Thus, in the electronic shell of the peptide bond, constructed in accordance with the theory of Snelson, there are no fundamental inconsistencies with generally accepted ideas about its structure.
The construction of ring-shaped electron shells, apparently, is applicable also for modeling the spatial structure of proteins, but for large molecules it is more convenient to use not 3D-like and mock-ups, but computerized 3D-modeling. Attempts at such a simulation are discussed in detail in a recent monograph / 7 /.
Another proof of the correctness of Snelson's theory can be built on the prototyping of the electronic shells of the four bases of DNA. And on the demonstration of the presence of hydrogen bonds in pairs of complementary bases AT and GC.
When describing the electron shells of these nitrogenous bases, it is desirable to follow the generally accepted numbering of the pyrimidine atoms (C and T) and the purine (A and G) nitrogenous bases shown in the figure.
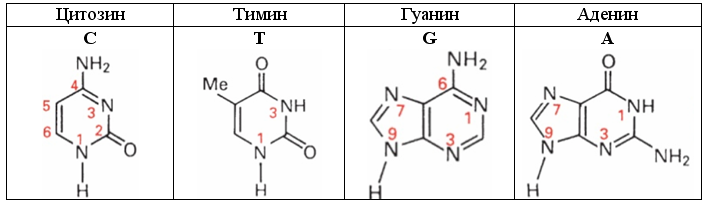
Fig. 26. DNA bases of DNA
A characteristic feature of the structure of bases C, T, G and A, as well as their pairs in DNA, is planarity. It follows that at least some of the bonds of nitrogen atoms in these bases can be either identical or similar to the planar peptide bond CO-N.
The planarity of bonds is also characteristic of benzene-forming ten-electron pairs -CH = CH-and -CH = N-, which are part of such benzene analogues as pyrimidine and pyrazine. There are two ten electron pairs of atoms of the benzene type in cytosine (C 5 -C 6 , N 3 -C 4 ), in thymine one (C 5 -C 6 ), in adenine, four (C 4 -C 5 , N 1-C 6 , C 2 -N 3 , N 7 -C 8 ) and guanine has three (C 4 -C 5 , C 2 -N 3 , N 7 -C 8 ).
It is easy to get confused in various bonds of purine and pyrimidine heterocycles, therefore it is better to consider them on electronic shells.

Fig. 27. Dummies of the electron shells of nitrogenous bases (the oxygen atom of deoxyribose is indicated by an eight-electron shell of transparent cringles)
At least five types of interatomic bonds are found in annular electron shells of nitrogenous bases:
• electronic (lonsdalelit type, with interatomic electron insertion);
• hole (without electronic insertion);
• benzene (–C = C– and –N = C– bonds with pairs of five-electron shells);
• ten-electron (CO);
• fan (four electronic rings, coupled at one point).
For example, cytosine has four electronic bonds (N 1 -C 2 , C 4 -C 5 , C 4 - (NH2 ) and C 6 -N 1 ), one hole (N 1 -dRib), two benzene (N 3 -C 4 , C 5 -C 6 ), one ten-electron (C 2 O) and one fan-shaped (C 2 -N 3 ). Thymine has four electronic bonds (N 1 -C 2 , C 2 -N 3 , N 3 -C 4 and C 6 -N 1 ), two hole (C 5 - (CH 3 )) and N 1 -dRib), one benzene (C5 -C 6 ), two ten-electron (C 2 O, C 4 O) and one fan-shaped (C 4 -C 5 ). All this, of course, is interesting, but it would be more interesting to look at the presence of hydrogen bonds in complementary pairs of nitrogenous bases.
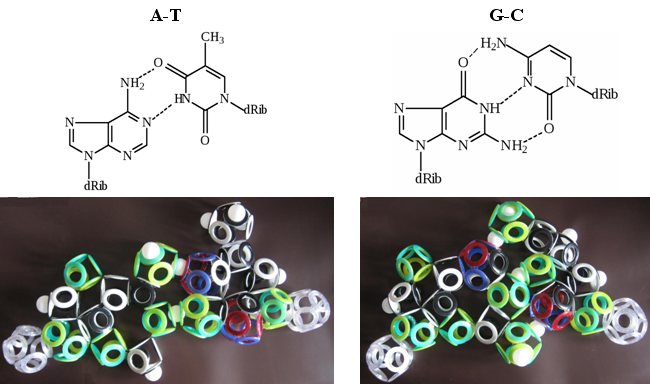
Fig. 28. Complementarity of nitrogenous bases
On the cringling models of the Snelson electronic shells, two hydrogen bonds in the AT pair and three in the GC are clearly visible. This is hardly a coincidence. And for those who understand the intricacies of the structure of DNA, this can serve as undeniable proof of the correctness of Kenneth Snelson's theory. The only stumbling block for them will be the magnetic isomerism of the electron shells of all biomolecules, which does not fit into modern ideas about their structure.
Magnetic isomers of biomolecules can hardly be distinguished by any physical methods, but their organoleptic properties should differ greatly, as can be demonstrated by the example of magnetic isomers of ethanol (C 2 H 5 OH).

Fig. 29. Magnetic isomers of ethanol.
As is well known, even the highest purification of ethanol, diluted to a standard concentration of 40%, is almost impossible to drink without snack and in uncooled form. But fresh moonshine of the same concentration is drunk even warm. Snack in this case will not be superfluous, but if you drink a little, you can do without it. Any chemist with elementary equipment is able to verify this. And not even a chemist, if he prefers to rely on his own experience and has a moonshine.
Snelson's theory makes it possible to explain this fact by preserving the magnetic isomerism of ethanol in fresh moonshine and the racemization of its molecules in purified alcohol, but quantum mechanics is powerless here. But one should not dissuade quantum mechanics experts with such examples. Do not understand.
The theory of the electron shells of atoms and molecules, proposed by Kenneth Snelson, can hardly compete in the near future with quantum-mechanical concepts based on a coherent system of postulates. The main postulate of this system - the principle of uncertainty of Heisenberg - could not even be refuted by Einstein, therefore, when meeting with experts on quantum mechanics, do not try to argue with them. And try not to smile. It annoys others.
1. Kenneth Snelson; Art and Ideas / In association with the Marlborough gallery, NY, 2013, NY, 174 P. http://kennethsnelson.net/KennethSnelson_Art_And_Ideas.pdf
2. Kozhevnikov D.N. Creation and use of a complex of models of atoms and molecules to study the structure of matter in the course of secondary school chemistry. on competition uch. degree of candidate ped. Sciences, 2004. - 171 p.
3. Hyman R. B., Evsyukov S. E. Allotropy of carbon // Nature. 2003. - №8. - pp.66-72.
4. Sladkov A.M., Kudryavtsev Yu.P. Almaz, graphite, carbin - allotropic forms of carbon // Nature. - 1969. - № 5. - p.37-44.
5. Ivanov D.M., Ivanov M.G. Chemistry of halogens // Educational edition. - 2014. - Publishing house of the Ural University. - 82 s.
6. Ovchinnikov Yu.A. Bioorganic chemistry // 1987. - M .: publishing house "Enlightenment", 814 p.
7. Sokolik V.V., Kushelev A.Yu. Geometry of the living nanoworld. Protein picotechnology / Lambert Academic Publishing, 2016. - 292 p.
PS: Version of the article in PDF format can be viewed here .

Fig. 1. “Atomic” sculptures (4'x4'x4 ', granite, 2009) / 1 /
Electrons in the atomic model of Snelson have a ring shape and form spherical electron shells consisting of contiguous electron rings (“circle-sphere”). Models of such "cyclospheres" Snelson built from ferrite ring magnets. If they are located on the surface of a sphere, then, when the direction of the magnetic field alternates, the edges of the adjacent magnets attract each other, and their outer planes form polyhedral (ring-bound) shells.
')

Fig.2. Magnetic models of Snelson electron shells
The most stable "electronic" structures are obtained from two, eight, ten and fourteen magnets.
Periodic table according to Snelson
According to Snelson's theory, such stable combinations of magnets correspond to the four main types of filled atomic shells constructed from ring electrons. The first type (2e - ) corresponds to helium, the atomic radius of which is 31 pm. The second (8e - ) is characteristic of noble gases, closing the periods in the periodic table (Ne - 38 pm; Ar - 71 pm; Kr - 88 pm; Xe - 108 pm; Rn - 120 pm). Larger filled shells (10e - ) have nickel subgroup elements (Ni - 149 pm; Pd - 169 pm; Pt - 177 pm). A shell of 14 electrons can form in lanthanides (Er - 226 pm) and, possibly, in actinides. In this case, the grouping of chemical elements according to the types of external electron shells of atoms reflects their division into small (8e - ) and large (10e - + 8e - or 14e - + 10e - + 8e - ) periods of the Periodic Table.

Fig. 3. Periodic table with division of periods according to Snelson
The position of some elements in this table looks strange. For example, sodium, rubidium and cesium, despite the similarity of chemical properties, turn out to be in different series of colors - yellow (8e - ), blue (10e - ) and lilac (14e - ). Copper, silver and gold are colored the same way as lithium and sodium are yellow. But keep in mind that the external electrons of elements from lithium to neon and from sodium to argon form eight-electron shells, and from potassium to nickel and from copper to krypton - ten-electron. On the same basis, elements from copper to krypton, from silver to xenon and from gold to radon are highlighted in yellow, and blue from rubidium to palladium and from thulium to platinum.
In this case, the chemical properties of elements with one electron on the outer shell are determined by the type of the underlying filled electron shell — eight-electron for alkali metals and ten-electron for copper, silver, and gold.
The radii of atoms in the periodic table change periodically — they increase spasmodically in the transition to each subsequent period, but within periods they decrease.

Fig. 4. Periods of atomic radii reference (with modifications)
From this it follows that the radii of ring electrons in atoms are also variable and depend on the distance to the atomic nucleus, its charge, and the type of electron shell. They are maximal on the outer shell, and are especially large at the beginning of the period when the charge of the atomic nucleus (for a given period) is minimal. In this case, the type of the filled electron shell depends on the atomic radius. If it is not more than 120 pm, then eight-electron shells are obtained. If more than 140 pm, then ten-electron. The construction of a filled shell for larger lanthanides (> 200 pm) requires no less than 14 electrons.

Fig. 5. Atomic radii with filled electron shells ( reference )
In the periodic table with periods separated by Snellson, the position of noble gases with completed eight-electron shells looks quite logical. It is logical to look at it and the elements with filled ten-electron shells. These are silvery metals characterized by high chemical resistance and plasticity (nickel, palladium and platinum). But the construction of the 14-electron shell of lanthanides should be completed in erbium, which does not differ in any way from the preceding dysprosium and holmium, or from the thulium, yttrium and lutetium that follow it. But on going from lutetium to hafnium, there is a sharp increase in density - from 9.85 to 13.2 g / cm3. Apparently, only at this stage, due to a decrease in the atomic radius, it becomes possible to form a stable 14-electron shell. And with its formation, there are "extra" external electrons. Therefore, in nature, there is no element with a 14-electronic complete outer shell that is noticeably different in chemical properties from neighboring elements.
The cyclosphere theory of the structure of the electron shells of atoms, proposed by Snelson, is in good agreement with the periods of the periodic table, so it would be interesting to evaluate its consistency with the properties of molecules.
Eight-electron shells of the simplest molecules
The outer shell of eight electrons, characteristic of noble gases, may have even the simplest molecules containing hydrogen. For example, a methane molecule (CH 4 ). Usually in its models hydrogen is designated by a hemisphere or a separate ball. In the cyclospheric (ring-shaped) model, the eight-electron shell of methane consists of four ring electrons of the outer shell of a carbon atom and four hydrogen atoms (protonated electrons).

Fig. 6. Models of methane molecule
The attraction of “hydrogen” electrons to protons reduces their diameters, and the repulsion of protons from a positively charged nucleus slightly pushes them out of the plane of the electron rings. But if you make the electrons invisible and connect the carbon nucleus to the protons by lines, then the ring-grit model of the methane molecule will take on a classic appearance - symmetrical, with the same angles (109.5 °) between all hydrogen atoms.

Fig. 7. The structure of the methane molecule
Ring-bound electron shells of the borohydride anion (BH 4 - ) and the ammonium cation (NH 4 + ) may look similar. But the borohydride should be slightly larger, and the ammonium should be slightly less methane.
The ammonia molecule (NH 3 ) also has an eight-electron shell, although the loss of one ammonium proton deprives it of a positive charge, breaks the symmetry and slightly changes the angles of location of the three remaining protons. And the same electron shell, but slightly smaller in diameter, should be near the hydroxonium cation (H 3 O + ).
By the same principle, it is possible to construct an eight-electron shell of a water molecule, in which the angle formed by the oxygen nucleus and two protons will be even smaller.

Fig. 8. Models of ammonia and water molecules / 2 / ( picture )
Thus, it is possible to identify a number of simplest molecules and ions with eight-electron shells, in which the shortage of external electrons is compensated by hydrogen atoms (protonated electrons). For the elements of the second period, these are BH 4 - , CH 4 , NH 4 + , NH 3 , H 3 O + , H 2 O, OH - , HF and F - . Neon closes the second period, which does not require protons to neutralize the total negative charge of electrons of the outer shell of an atom.
By the same principle, external eight-electron shells are constructed for SiH 4 , PH 3 , SH 2 and HCl molecules, formed by elements of the third period of the Periodic Table. The hydrogen atoms involved in the construction of the electron shells of such molecules and ions affect their shape and chemical properties, but do not change the overall structure.
In all such models, the interposition of heavy nuclei with an inner electron shell and “bare” protons is in good agreement with the well-known “canonized” structures of these simplest molecules. And according to Snelson's theory, the interposition of the central nucleus and protons is explained by the stability of the eight-electron outer shells of similar molecules formed by atoms of the second and third periods of the Periodic Table.
The similarities and differences in the electron shells of such simplest molecules well reflect (and explain well) their known structural parameters, but with water molecules everything can be more complicated. In addition to the usual "meta-water", in which the protons are located at an angle of 104.5 °, there must be a "steam-water" in which the protons lie on the same straight line with the nucleus of oxygen (180 °). Moreover, the "meta-water" nuclei of hydrogen atoms (protons) can be either in the "northern" (N) or in the "southern" (S) electrons. In "para-water" such magnetic isomerism is absent, since its proton-containing electrons have opposite (relative to the center of the molecule) orientation of the magnetic field.
The structural and magnetic isomerism of the electron shells of water molecules in cyclospheric models contradicts its generally accepted descriptions. This could serve as proof of the fallacy of Snelson's theory, if it were not for the surprising diversity of the properties of water, which is not explained within the framework of generally accepted ideas about the only variant of its molecular structure.
Magnetic isomerism should be characteristic of electron shells and many other molecules. But this topic, as well as the theoretical justification of the properties of water, it is better to discuss separately.

Fig. 9. Magnetic isomers of the simplest molecules (cringling models of electron shells)
Separate consideration is given to the numerous carbon compounds in which the electron shells should differ noticeably from the shells of the simplest molecules described above.
Electron shells of carbon compounds
For compounds consisting only of carbon, the extreme diversity of molecular structures that form materials with unique properties (carbin, graphene, fullerene, nanotubes, diamond, etc. / 3 /) is characteristic. After awarding the Nobel Prizes to the discoverers of fullerene (1996; R. Curl, H. Kroto, R. Smolli) and graphene (2010; AK Geim, KS Novoselyov), predictions and searches for new forms became more popular carbon. As a result of such predictions, molecules of graphite, grafdin, phagrafen, and other predominantly hypothetical carbon compounds have been described.
The simplest arrangement is carbyne - a linear polymer of carbon / 4 /. Assembling a model of its electron shell is not difficult, since there are four electrons on the outer shell of a carbon atom. These electrons form a "cube" with two unfilled faces. The assembly of such "cubes" of the linear polymer gives carbin.

Fig. 10. Carbin
The high fragility of carbyne allows it to bend and form closed rings. Due to the obligatory alternation of the poles of the magnetic field in adjacent electrons of the ring, there can be only an even number of carbon atoms. Rings with six carbon atoms (hexamers, C 6 ), whose electrons can connect at different angles and form electron shells of various types, should be especially stable. The most famous structures form the "Nobel" molecules - graphene and fullerene. It is not difficult to construct for them models of electron shells of the Snelson type, if we use different types of hexamer carbon rings, such as graphene (2D) and fullerene (3D), in this construction.

Fig. 11. Main 2D and 3D carbon forms
Fullerenes got their name in honor of the outstanding American architect, designer and inventor Richard Fuller. But a great contribution to the theory of the famous Fuller was made by his less famous employee and not less talented inventor Kenneth Snelson. Therefore, it would be nice to give his still undiscovered carbon molecules its name. Moreover, the Snelson theory allows not only to describe the already known forms of carbon, but also to predict its yet unknown varieties. Many such structures can be built, but, as a rule, they are distinguished not only by increased flexibility and fragility, but also the ability to form cohesive three-dimensional conglomerates, which makes it difficult to separate individual chemicals from a mixture of similar structural analogs.
It is also possible to build electron shells of the Snelson type for the most durable form of carbon - diamond. And not only for its usual variety, but also for a more rare and poorly studied form - lonsdaleite.

Fig. 12. The structure of the diamond electron shells
The diamond crystal lattice can be considered a three-dimensional polymer of methane, in which all hydrogen atoms are replaced by carbon-carbon "hole" bonds. Each carbon atom is surrounded by four electrons and four interatomic bonds, with small gaps between the electron shells of individual atoms. In addition, each electron is linked with six neighboring electrons.
In a lonsdaleite crystal, carbon atoms have eight-electron shells, and all the electrons forming them serve as interatomic septa (“electronic” bonds). As a result, for each carbon atom there are four “whole” electrons. More precisely - eight of their "halves", divided between adjacent atoms. Unlike ordinary diamond, lonsdaleite has no interatomic gaps. In addition, each electron is also associated with six neighboring electrons.
Similar variants of the structure of the electron shells can have crystals and other particularly hard substances — moissanite (SiC), borazone (γ-BN), and boron phosphide (BP). The high hardness of all such compounds is well explained by the structure of their electron shells, in which each electron is associated with six neighbors. In ordinary molecules, electrons have no more than four such bonds.
The Snelsonian theory of the structure of molecular electron shells rather well explains the diversity of allotropic forms of carbon, but to prove its universality, it is advisable to test this theory on other carbon compounds. First of all - on well-studied hydrocarbons.
Hydrocarbon electron shells
Alkanes, i.e. saturated hydrocarbons with the general formula C n H 2n + 2 can be considered derivatives of methane, in which some proton electrons are replaced by “hole” carbon-carbon bonds. Three of the simplest molecules of this type include ethane, propane, butane and isobutane.

Fig. 13. Models of electronic shells of simplest alkanes
For propane and other alkanes having an odd number of carbon atoms in an unbranched chain, magnetic isomerism is characteristic. In linear saturated hydrocarbons with an even number of carbon atoms, magnetic isomerism is absent. And with an increase in the number of carbon atoms in a molecule, the number of magnetic, structural and rotational isomers (rotamers) increases exponentially.

Fig. 14. Magnetic, structural and rotational isomers of pentane
Even more isomers can be constructed for hexane, but this does not apply to cyclic saturated hydrocarbons. For example, cyclohexane has no magnetic isomers, although if we look at the model of its electron shell from the side, we can see that the unidirectional spins of the upper and lower electrons have the opposite orientation.

Fig. 15. Model of the electron shell of cyclohexane (C 6 H 12 ) (two sides of the same "medal")
The original electron shell has a benzene molecule (C 6 H 6 ) containing six symmetric planar hydrogen atoms. It is impossible to build such a shell from the hexamer C 6 , characteristic of graphene or fullerene. But it is easily built on the basis of a ring of six interlinked electrons, forming a central hexagonal cylinder. In this construction, for each carbon atom there are five electrons - four of its own and one "hydrogen". This structure explains both the low density of benzene (0.876 g / cm ³), the symmetry of the structure of the molecule, the planarity of the arrangement of hydrogen atoms, and their ability to be replaced by radicals, which lack one electron to complete the octahedral shells.

Fig. 16. Electron shell of a benzene molecule
Typical for benzene carbon atoms, the five-member compounds of ring electrons are repeatedly found in Snelson's paintings, and it would be strange if they were absent in chemical compounds.

Fig. 17. Five-electron shells of Snelson / 1 /
Based on the above described version of the electron shell of benzene, it is possible to construct shells for its numerous derivatives with various side radicals, as well as with nitrogen atoms, partially or completely replacing the central ring carbon.

Fig. 18. Models of electron shells of simplest benzene derivatives
The outer electron shell of ethylene (C 2 H 4 ) —the simplest representative of alkenes (C n H 2n ) —is easily constructed from eight ordinary and four protonated electron rings. Analogous 12-electron shells can have molecules of diimide (N 2 H 2 ), oxygen (O 2 ) and formaldehyde (H 2 O).

Fig. 19. Mock-ups of molecules with 12-electron shells.
All other alkenes, dienes and trienes can be considered alkanes containing one, two or three ethylene groups.
Hydrocarbons belonging to alkynes (C n H 2n-2 ) contain a pair of carbon atoms combined by an elongated 10-electron shell. For each of the carbon atoms there are four electrons; therefore, the missing positions of acetylene are built up with two hydrogen atoms, and for the remaining alkynes, these hydrogens are replaced by one or two “hole” bonds with the electron shells of neighboring atoms.

Fig. 20. Dummy shells of simplest alkynes
Thus, for all major types of hydrocarbons — alkanes, arenes, alkenes, and alkynes — models of external electron shells can be constructed that satisfy all the canons of Kenneth Snelson's theory. Moreover, such shells can serve as samples for molecular prototyping of compounds containing not only carbon atoms. Illustrative examples are the 12-electron shells of ethylene analogs - diimide, oxygen and formaldehyde described above. By analogy with them, it would be interesting to consider the Snelsonian shells of more complex molecules containing 10-electronic structures of acetylene type.
Molecules with ten-electron shells
The spherical shell, built of ten electronic rings, is capable of stretching in different directions. Best of all - along the axis of symmetry passing through a pair of electrons at the "poles". In chemically unstable acetylene (C 2 H 2 ), this axis passes through hydrogen atoms. In the chemical resistance of molecular nitrogen (N 2 ), hydrogen atoms are absent, and the elongated electron shell is characterized by increased strength. A similar Snelson 10-electron shell can be constructed for some other simplest molecules.

Fig. 21. Layouts of 10-electron shells of simplest molecules
More complex electronic shells containing inserts of 10-electronic elements may have molecules of urea, acetone, acetamide, carbonic and acetic acids, as well as guanidine. A characteristic feature of all molecules of this type is their magnetic isomerism and the presence of interatomic bonds with electronic inserts ("electronic" bonds).

Fig. 22. Layouts of molecular shells with 10-electron central groups.
Especially often in organic compounds occurs 10-electron group of carbon monoxide (CO). Some heterocyclic compounds contain two, three or even four such groups.

Fig. 23. Examples of heterocycles with 10-electron groups WITH
A characteristic feature of the electron shells of such molecules is the presence of interatomic bonds of the lonsdaleite type (with insertions of interatomic electrons). Similar "electronic" bonds may be present in many other molecules. For example, in polyiodides (I 3 - , I 5 - , I 7 - , I 9 - and others), which crystallize from solutions containing iodides (I - ) and iodine (I 2 ) / 5 /.

Fig. 24. Layout of the electron shell of anion I 3 - with interatomic bonds of lonsdailite type
It would be very interesting to analyze the structure of the electron shells of numerous halogen compounds, but only for a limited circle of chemists. Therefore, it is better to check the correctness of the theory proposed by Snelson by constructing the electron shells of more well-known molecules - proteins, DNA, etc ...
Biomolecules
The main structural element of all protein molecules is a peptide bond. The coincidence of its known parameters with the parameters of a ring-shaped electron shell can serve as a good proof of the correctness of Snelson's theory. A mismatch - a refutation.

Fig. 25. Peptide Coupling
The cringing model displays only the electron shell, which does not allow to accurately determine the angles between the centers of the atoms. However, their general arrangement is consistent with the schemes from textbooks / 6 /. For example, the layout clearly shows the planarity of the three bonds of the peptide nitrogen atom, which in the majority of its compounds are located pyramidally. Such a planarity can be explained by the presence of an interatomic electron between the 10-electron shell of CO and the octahedral shell of the nitrogen atom.This electron is adjacent to the unfilled electron ("hole") bond NC (indicated by a transparent grommet), and the most stable position for the protonated electron (hydrogen atom) in this case becomes the opposite face of the shell of the nitrogen atom.
The decrease in the peptide bond length (CO) -N (1.32 Å) compared with the length of the single CN bond (1.47 Å) can also be explained by the presence of an interatomic ("lonsdailite") electron in it. Thus, in the electronic shell of the peptide bond, constructed in accordance with the theory of Snelson, there are no fundamental inconsistencies with generally accepted ideas about its structure.
The construction of ring-shaped electron shells, apparently, is applicable also for modeling the spatial structure of proteins, but for large molecules it is more convenient to use not 3D-like and mock-ups, but computerized 3D-modeling. Attempts at such a simulation are discussed in detail in a recent monograph / 7 /.
Another proof of the correctness of Snelson's theory can be built on the prototyping of the electronic shells of the four bases of DNA. And on the demonstration of the presence of hydrogen bonds in pairs of complementary bases AT and GC.
When describing the electron shells of these nitrogenous bases, it is desirable to follow the generally accepted numbering of the pyrimidine atoms (C and T) and the purine (A and G) nitrogenous bases shown in the figure.

Fig. 26. DNA bases of DNA
A characteristic feature of the structure of bases C, T, G and A, as well as their pairs in DNA, is planarity. It follows that at least some of the bonds of nitrogen atoms in these bases can be either identical or similar to the planar peptide bond CO-N.
The planarity of bonds is also characteristic of benzene-forming ten-electron pairs -CH = CH-and -CH = N-, which are part of such benzene analogues as pyrimidine and pyrazine. There are two ten electron pairs of atoms of the benzene type in cytosine (C 5 -C 6 , N 3 -C 4 ), in thymine one (C 5 -C 6 ), in adenine, four (C 4 -C 5 , N 1-C 6 , C 2 -N 3 , N 7 -C 8 ) and guanine has three (C 4 -C 5 , C 2 -N 3 , N 7 -C 8 ).
It is easy to get confused in various bonds of purine and pyrimidine heterocycles, therefore it is better to consider them on electronic shells.

Fig. 27. Dummies of the electron shells of nitrogenous bases (the oxygen atom of deoxyribose is indicated by an eight-electron shell of transparent cringles)
At least five types of interatomic bonds are found in annular electron shells of nitrogenous bases:
• electronic (lonsdalelit type, with interatomic electron insertion);
• hole (without electronic insertion);
• benzene (–C = C– and –N = C– bonds with pairs of five-electron shells);
• ten-electron (CO);
• fan (four electronic rings, coupled at one point).
For example, cytosine has four electronic bonds (N 1 -C 2 , C 4 -C 5 , C 4 - (NH2 ) and C 6 -N 1 ), one hole (N 1 -dRib), two benzene (N 3 -C 4 , C 5 -C 6 ), one ten-electron (C 2 O) and one fan-shaped (C 2 -N 3 ). Thymine has four electronic bonds (N 1 -C 2 , C 2 -N 3 , N 3 -C 4 and C 6 -N 1 ), two hole (C 5 - (CH 3 )) and N 1 -dRib), one benzene (C5 -C 6 ), two ten-electron (C 2 O, C 4 O) and one fan-shaped (C 4 -C 5 ). All this, of course, is interesting, but it would be more interesting to look at the presence of hydrogen bonds in complementary pairs of nitrogenous bases.

Fig. 28. Complementarity of nitrogenous bases
On the cringling models of the Snelson electronic shells, two hydrogen bonds in the AT pair and three in the GC are clearly visible. This is hardly a coincidence. And for those who understand the intricacies of the structure of DNA, this can serve as undeniable proof of the correctness of Kenneth Snelson's theory. The only stumbling block for them will be the magnetic isomerism of the electron shells of all biomolecules, which does not fit into modern ideas about their structure.
Magnetic isomers of biomolecules can hardly be distinguished by any physical methods, but their organoleptic properties should differ greatly, as can be demonstrated by the example of magnetic isomers of ethanol (C 2 H 5 OH).

Fig. 29. Magnetic isomers of ethanol.
As is well known, even the highest purification of ethanol, diluted to a standard concentration of 40%, is almost impossible to drink without snack and in uncooled form. But fresh moonshine of the same concentration is drunk even warm. Snack in this case will not be superfluous, but if you drink a little, you can do without it. Any chemist with elementary equipment is able to verify this. And not even a chemist, if he prefers to rely on his own experience and has a moonshine.
Snelson's theory makes it possible to explain this fact by preserving the magnetic isomerism of ethanol in fresh moonshine and the racemization of its molecules in purified alcohol, but quantum mechanics is powerless here. But one should not dissuade quantum mechanics experts with such examples. Do not understand.
Conclusion
The theory of the electron shells of atoms and molecules, proposed by Kenneth Snelson, can hardly compete in the near future with quantum-mechanical concepts based on a coherent system of postulates. The main postulate of this system - the principle of uncertainty of Heisenberg - could not even be refuted by Einstein, therefore, when meeting with experts on quantum mechanics, do not try to argue with them. And try not to smile. It annoys others.
BIBLIOGRAPHY
1. Kenneth Snelson; Art and Ideas / In association with the Marlborough gallery, NY, 2013, NY, 174 P. http://kennethsnelson.net/KennethSnelson_Art_And_Ideas.pdf
2. Kozhevnikov D.N. Creation and use of a complex of models of atoms and molecules to study the structure of matter in the course of secondary school chemistry. on competition uch. degree of candidate ped. Sciences, 2004. - 171 p.
3. Hyman R. B., Evsyukov S. E. Allotropy of carbon // Nature. 2003. - №8. - pp.66-72.
4. Sladkov A.M., Kudryavtsev Yu.P. Almaz, graphite, carbin - allotropic forms of carbon // Nature. - 1969. - № 5. - p.37-44.
5. Ivanov D.M., Ivanov M.G. Chemistry of halogens // Educational edition. - 2014. - Publishing house of the Ural University. - 82 s.
6. Ovchinnikov Yu.A. Bioorganic chemistry // 1987. - M .: publishing house "Enlightenment", 814 p.
7. Sokolik V.V., Kushelev A.Yu. Geometry of the living nanoworld. Protein picotechnology / Lambert Academic Publishing, 2016. - 292 p.
PS: Version of the article in PDF format can be viewed here .
Source: https://habr.com/ru/post/374003/
All Articles