What holds the nuclei of atoms?
Now that we know that the nucleus of an atom is tiny , we have an obvious question: why is it so small? Atoms are made up of tiny particles, but they are much larger in size than these particles . We have already figured out why this is happening. But at the same time, the nuclei are not very different in size from the protons and neutrons of which they are composed. Is there a reason for this, or is this a coincidence?
We already know that atoms hold electrical forces . What forces hold the nucleus of the atom?
And here we are entering a new territory, which is very different from what we studied earlier - because it becomes obvious that a force is working here that we have not yet discussed.
')
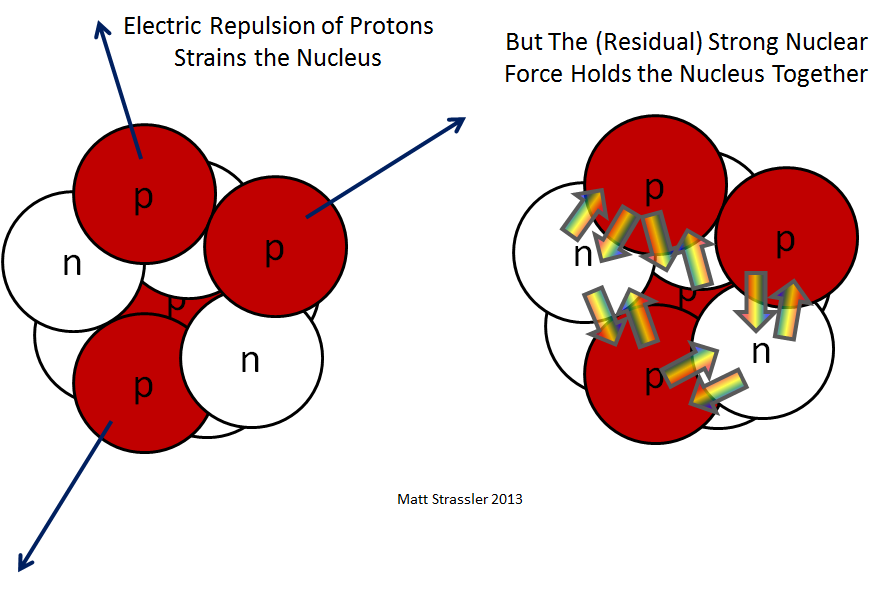
Fig. 1: the counteracting forces in the nucleus of the atom - the electric repulsion of protons and the residual strong nuclear interaction of protons and neutrons
If nature had only gravitational and electrical interactions that we encounter in everyday life, nuclei with many protons would simply scatter: electrical forces pushing protons apart would be a million million million times greater than their gravitational pull. So there must be another force that provides attraction, overpowering electrical repulsion. This force is a strong nuclear interaction — although in the core itself only a shadow of its true greatness can be observed. Having studied the structure of the protons and neutrons themselves, we will understand the true possibilities of a strong nuclear interaction. And in the core, we meet only what is often called “residual interaction” - and I will call it “residual strong nuclear interaction”. Sometimes this term is not used - it is simply called a strong nuclear interaction, but such a distinction is useful.
Warning: in the end, it turns out that although, in general, a strong nuclear interaction - a force acting between particles (quarks, gluons, antiquarks) inside a proton or neutron - is quite simple, in a sense, the residual strong nuclear interaction is a complex residue of various mutually destroyed effects, and therefore there is no simple picture that describes the whole physics of the nucleus. And this is not surprising, given the internal complexity of the structure of protons and neutrons. Here you can draw some kind of analogy between atoms and molecules.
In the atom, the tiny nucleus and even smaller electrons are located far from each other with respect to their size, and the electrical forces that keep them in the atom are simple. But in molecules, the distance between atoms is comparable to the size of atoms, so the internal complexity of atoms begins to play a role. The variety and complex sets of partially compensating electrical forces, and the processes in which electrons are able to move between atoms, make the history of molecules much richer and more complex than that of atoms. Similarly, the distance between protons and neutrons in a nucleus is comparable to their size — therefore, as with molecules, the forces holding atomic nuclei turn out to be much more difficult (in certain senses) the forces holding protons or neutrons.
After studying the structure of protons and neutrons, this story will become (a little) clear. The basic properties of nuclear physics are quite clear, but this topic remains extremely technical, and many details are still being explored. I will not be able to properly describe it in this article, in particular, because I am not well-versed in this topic to conveniently simplify it for you.

Fig. 2: the lightest of stable and almost stable nuclei, along with a neutron. Neutrons and tritium are shown in dotted lines, since they ultimately decay. Blue color indicates alternative names.
Let's see what can be learned from simple reasoning about the work of this force. One of the clues is that all the nuclei, with the exception of the most common hydrogen isotope (one proton), contain neutrons; that is, there are no nuclei with several protons and without neutrons (Fig. 2). So clearly neutrons play an important role in helping protons stick together.
Conversely, there are no nuclei consisting of neutrons alone; in the lightest nuclei, for example, in oxygen or silicon, about the same number of neutrons as protons (Fig. 2). In larger and more massive nuclei, such as gold and radium, there are slightly more neutrons than protons (Fig. 3). Two things follow from this:
An illustration of the last statement is presented in Fig. 3, which shows stable (black) and relatively long-lived, but unstable (colored) nuclei, in the form of a graph of the dependence of the number of protons Z on the number of neutrons N contained in them. Note that in stable nuclei, Z and N are approximately equal for small values, but N gradually becomes larger than Z, with their increase. Also note that the band of stable and long-lived nuclei remains fairly narrow for all Z values. Despite the tremendous progress of nuclear physics over the past 80 years, there is no generally accepted and simple explanation for this remarkable fact. I think it is considered a strange coincidence.
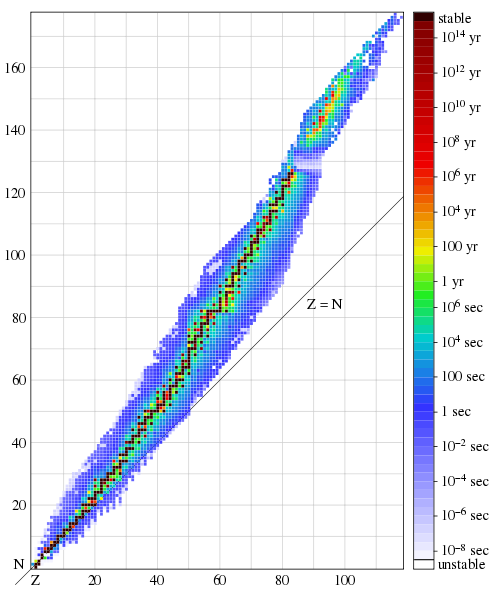
Fig. 3
One of the main goals of this article is to explain why the nuclei of atoms are small compared to the size of an atom. To do this, we start with the simplest nucleus containing protons and neutrons - the second most abundant isotope of hydrogen, consisting of one electron (like all hydrogen isotopes) and the nucleus consisting of a proton and a neutron. This isotope is often called deuterium, and the nucleus of deuterium (see Fig. 2) is sometimes called deuteron. How to understand what holds the deuteron? We can naively assume that this system does not differ from a hydrogen atom, which also contains two particles (proton and electron) - see fig. four.
As we saw in the previous article , the fact that the mass of electrons is small compared to protons and neutrons ensures that:
What about deuteron? It consists of two objects in a similar way, but of almost equal mass (the neutron and proton masses differ by only 1/1500, for reasons that we will understand later), so both of them are equally important in determining the mass and size of the deuteron. Suppose we would have a new force attracting a proton to a neutron, similar to an electromagnetic one (in fact, everything is different, but just imagine): then, by analogy with hydrogen, we would expect that the size of the deuteron would be inversely proportional to the mass of the proton or neutron, and inversely proportional to the strength of the new interaction. If this interaction were as strong at a certain distance as electromagnetism, it would mean that, since the proton is about 1850 times heavier than an electron, that the deuteron (and any nucleus) must be at least 1000 times less hydrogen.
But we already guessed that the residual strong interaction is stronger than electromagnetism at the same distance - because otherwise it could not prevent the electromagnetic repulsion of protons, which would have broken the nucleus. So this extra force will pull the protons and neutrons together even more tightly. It is therefore not surprising that the deuteron and other nuclei are not just a thousand, but tens of thousands of times smaller than atoms! I repeat, this is all because:
This naive guess led us to almost the right answer! But it does not fully describe the complexity of the interactions between the proton and the neutron in deuterium. One obvious problem is a force similar to electromagnetism, but greater power would obviously affect everyday life, and we are not seeing anything like that. So something about this interaction should be different from the electric one.
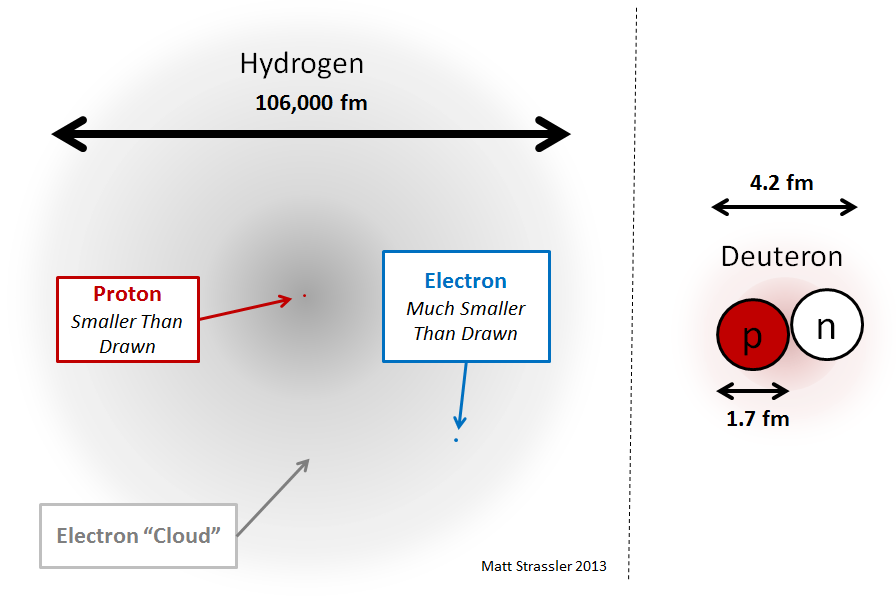
Fig. four
The difference is that this residual strong nuclear interaction is very important and powerful for protons and neutrons located very close to each other, but at rather large distances (at a distance of the force) it starts to decrease very quickly, much faster than electromagnetic. The distance, by some coincidence, turns out to be equal to the size of a relatively large nucleus, only several times larger than a proton. If you bring a proton and a neutron together, at a distance comparable to this distance, they will attract each other and form a deuteron. If you leave them at a greater distance, they generally barely feel the attraction. And if you bring them very close together, so that they overlap each other, they will start to build on; damn, I warned you that the residual strong nuclear interaction is very complicated! Shortly speaking:
Larger nuclei are held more or less by the same interaction that holds the deuteron, but the details of this process are complex and technical, and they are not easy to describe. Yes, they are not yet fully understood. Although the general outlines of nuclear physics have been well understood for many decades, many important details are still being explored.
We already know that atoms hold electrical forces . What forces hold the nucleus of the atom?
And here we are entering a new territory, which is very different from what we studied earlier - because it becomes obvious that a force is working here that we have not yet discussed.
')

Fig. 1: the counteracting forces in the nucleus of the atom - the electric repulsion of protons and the residual strong nuclear interaction of protons and neutrons
Residual strong nuclear interaction
If nature had only gravitational and electrical interactions that we encounter in everyday life, nuclei with many protons would simply scatter: electrical forces pushing protons apart would be a million million million times greater than their gravitational pull. So there must be another force that provides attraction, overpowering electrical repulsion. This force is a strong nuclear interaction — although in the core itself only a shadow of its true greatness can be observed. Having studied the structure of the protons and neutrons themselves, we will understand the true possibilities of a strong nuclear interaction. And in the core, we meet only what is often called “residual interaction” - and I will call it “residual strong nuclear interaction”. Sometimes this term is not used - it is simply called a strong nuclear interaction, but such a distinction is useful.
Warning: in the end, it turns out that although, in general, a strong nuclear interaction - a force acting between particles (quarks, gluons, antiquarks) inside a proton or neutron - is quite simple, in a sense, the residual strong nuclear interaction is a complex residue of various mutually destroyed effects, and therefore there is no simple picture that describes the whole physics of the nucleus. And this is not surprising, given the internal complexity of the structure of protons and neutrons. Here you can draw some kind of analogy between atoms and molecules.
In the atom, the tiny nucleus and even smaller electrons are located far from each other with respect to their size, and the electrical forces that keep them in the atom are simple. But in molecules, the distance between atoms is comparable to the size of atoms, so the internal complexity of atoms begins to play a role. The variety and complex sets of partially compensating electrical forces, and the processes in which electrons are able to move between atoms, make the history of molecules much richer and more complex than that of atoms. Similarly, the distance between protons and neutrons in a nucleus is comparable to their size — therefore, as with molecules, the forces holding atomic nuclei turn out to be much more difficult (in certain senses) the forces holding protons or neutrons.
After studying the structure of protons and neutrons, this story will become (a little) clear. The basic properties of nuclear physics are quite clear, but this topic remains extremely technical, and many details are still being explored. I will not be able to properly describe it in this article, in particular, because I am not well-versed in this topic to conveniently simplify it for you.

Fig. 2: the lightest of stable and almost stable nuclei, along with a neutron. Neutrons and tritium are shown in dotted lines, since they ultimately decay. Blue color indicates alternative names.
Scheme of work
Let's see what can be learned from simple reasoning about the work of this force. One of the clues is that all the nuclei, with the exception of the most common hydrogen isotope (one proton), contain neutrons; that is, there are no nuclei with several protons and without neutrons (Fig. 2). So clearly neutrons play an important role in helping protons stick together.
Conversely, there are no nuclei consisting of neutrons alone; in the lightest nuclei, for example, in oxygen or silicon, about the same number of neutrons as protons (Fig. 2). In larger and more massive nuclei, such as gold and radium, there are slightly more neutrons than protons (Fig. 3). Two things follow from this:
- For protons to stick together, neutrons are needed, and for neutrons to stick together, protons are needed.
- If the number of protons and neutrons becomes very large, then the electric repulsion of protons must be compensated for by adding a few extra neutrons.
An illustration of the last statement is presented in Fig. 3, which shows stable (black) and relatively long-lived, but unstable (colored) nuclei, in the form of a graph of the dependence of the number of protons Z on the number of neutrons N contained in them. Note that in stable nuclei, Z and N are approximately equal for small values, but N gradually becomes larger than Z, with their increase. Also note that the band of stable and long-lived nuclei remains fairly narrow for all Z values. Despite the tremendous progress of nuclear physics over the past 80 years, there is no generally accepted and simple explanation for this remarkable fact. I think it is considered a strange coincidence.

Fig. 3
Core size
One of the main goals of this article is to explain why the nuclei of atoms are small compared to the size of an atom. To do this, we start with the simplest nucleus containing protons and neutrons - the second most abundant isotope of hydrogen, consisting of one electron (like all hydrogen isotopes) and the nucleus consisting of a proton and a neutron. This isotope is often called deuterium, and the nucleus of deuterium (see Fig. 2) is sometimes called deuteron. How to understand what holds the deuteron? We can naively assume that this system does not differ from a hydrogen atom, which also contains two particles (proton and electron) - see fig. four.
As we saw in the previous article , the fact that the mass of electrons is small compared to protons and neutrons ensures that:
- The mass of an atom is almost equal to the mass of its nucleus,
- The size of the atom (the size of the electron cloud) is inversely proportional to the mass of the electron and inversely proportional to the strength of the electromagnetic interaction; The uncertainty principle of quantum mechanics plays a critical role here.
What about deuteron? It consists of two objects in a similar way, but of almost equal mass (the neutron and proton masses differ by only 1/1500, for reasons that we will understand later), so both of them are equally important in determining the mass and size of the deuteron. Suppose we would have a new force attracting a proton to a neutron, similar to an electromagnetic one (in fact, everything is different, but just imagine): then, by analogy with hydrogen, we would expect that the size of the deuteron would be inversely proportional to the mass of the proton or neutron, and inversely proportional to the strength of the new interaction. If this interaction were as strong at a certain distance as electromagnetism, it would mean that, since the proton is about 1850 times heavier than an electron, that the deuteron (and any nucleus) must be at least 1000 times less hydrogen.
But we already guessed that the residual strong interaction is stronger than electromagnetism at the same distance - because otherwise it could not prevent the electromagnetic repulsion of protons, which would have broken the nucleus. So this extra force will pull the protons and neutrons together even more tightly. It is therefore not surprising that the deuteron and other nuclei are not just a thousand, but tens of thousands of times smaller than atoms! I repeat, this is all because:
- Protons and neutrons are almost 2000 times heavier than electrons,
- At such distances, the strong nuclear interaction between protons and neutrons of the nucleus is many times stronger than the corresponding electromagnetic forces (including the electromagnetic repulsion of protons in the nucleus).
This naive guess led us to almost the right answer! But it does not fully describe the complexity of the interactions between the proton and the neutron in deuterium. One obvious problem is a force similar to electromagnetism, but greater power would obviously affect everyday life, and we are not seeing anything like that. So something about this interaction should be different from the electric one.

Fig. four
Short distance action of this force
The difference is that this residual strong nuclear interaction is very important and powerful for protons and neutrons located very close to each other, but at rather large distances (at a distance of the force) it starts to decrease very quickly, much faster than electromagnetic. The distance, by some coincidence, turns out to be equal to the size of a relatively large nucleus, only several times larger than a proton. If you bring a proton and a neutron together, at a distance comparable to this distance, they will attract each other and form a deuteron. If you leave them at a greater distance, they generally barely feel the attraction. And if you bring them very close together, so that they overlap each other, they will start to build on; damn, I warned you that the residual strong nuclear interaction is very complicated! Shortly speaking:
- The residual strong nuclear interaction is much, much weaker than electromagnetism at distances much larger than the size of a typical nucleus, so that we do not encounter it in everyday life.
- At short distances, comparable to the nucleus, it becomes much stronger - this attraction (at not too small distances) is able to surpass the electrical repulsion of other protons.
Larger nuclei are held more or less by the same interaction that holds the deuteron, but the details of this process are complex and technical, and they are not easy to describe. Yes, they are not yet fully understood. Although the general outlines of nuclear physics have been well understood for many decades, many important details are still being explored.
Source: https://habr.com/ru/post/373979/
All Articles