Electrons: At the Back of Atoms
Electrons, tiny objects that inhabit the backstage of atoms, play a leading role in chemistry, carry electrical current through our electrical networks and inside lightning strikes, and make up the “cathode rays” used to create images in XX century television and on computer screens. This is the most typical example of (seemingly) elementary particles.
By “elementary,” I mean that electrons are indivisible and not composed of smaller particles. With the help of “seemingly” I remind that they are elementary, as far as we are allowed to judge this by modern knowledge - what we know about electrons was obtained in experiments, and our experiments do not have infinite power. If the electrons are not elementary, but so small that our current experiments cannot break them down - they will look elementary in all the experiments we conducted in the past and present, but not in all future experiments. So someday - after all, 80 years ago, people believed that protons could be elementary, but they lacked knowledge, and 150 years ago, people thought that atoms could be elementary, but they lacked knowledge - we may find that electrons are not elementary. But for now, since all the experiments available to us demonstrate that they are elementary, we will conditionally assume that this is the case - remembering that this is partly an experimental fact, and partly - an assumption!
The electron became the first of the detected subatomic particles (the first object found, whose size was smaller than an atom). At the time of its discovery, in the 1890s (usually written in 1897, but this discovery was somewhat gradual), scientific debates about whether matter consists of atoms, or whether atoms were just fiction, convenient for describing the behavior of matter, coming to an end. But even those who believed in the existence of atoms did not necessarily believe that atoms were indivisible (as their name implied, derived from the Greek “uncut”). A generation later, by the mid-1930s, physicists confirmed the existence of atoms, understood their basic structure, and learned how to calculate their properties with high accuracy. They carried out these calculations using equations from the theory of the behavior of matter of the 1920s, called "quantum mechanics", which became necessary because the famous Newton equations could not cope with the description of the work of atoms. Many key checks on the accuracy of quantum mechanics have been associated with accurate measurements of the behavior of electrons inside and outside of atoms.
All electrons are identical and indistinguishable; if I change two of them in places, you cannot detect it. So I can write about the "property of the electron", and you can be sure that these properties are for all electrons. What properties are inherent in them?
')
An electron has a mass - it is small compared to the mass of any atom, so you can usually forget about it in the elementary classes of chemistry, but it is not so small as to forget about it in particle physics and even in understanding the structure of atoms. Although electrons do not make a significant contribution to the mass of an atom, the mass of an electron is necessary to determine the size of an atom. This, in particular, is the importance of the field and the Higgs particle. This mass can be written in different ways, and each of the methods gives you a different perspective:
An electron has an electric charge - which means that it has electric and magnetic fields. An electrically charged force will act on an electrically charged particle in the presence of an electric field. It is these forces that keep electrons inside their atoms.
How big is the electric charge of an electron? Imagine static electricity - you walked along the carpet in your shoes, and then, touching the door handle, another person or computer (!!!), you will feel a spark. This spark transfers charge from one place to another - and usually it is 10 million million times the charge carried by an electron. Physicists measure charge using a randomly selected unit called pendant (just as time is measured in seconds and length in meters). A typical static electricity charge contains one millionth of a pendant. The magnitude of the electron charge is usually denoted by e, and e is approximately equal to 1.6 × 10 -19 C.
Electron size unknown; it may be a pointless object with no size, or it may have an extremely small size, the radius of which does not exceed 10 -18 m. It is at least 100,000,000 times smaller than the radius of an atom. Otherwise, we would see signs of electron size in experiments.
What does an electron actually look like? As I wrote in the article about atoms , it is difficult to define the concept of the size of an elementary particle, since an electron, although it is called a particle, is not some speck of dust or a grain of salt or sand. He also has wave properties. In an atom, electrons are in some sense distributed throughout the atom, as the sound wave propagates from the drum. In this sense, being inside an atom, they have the size of an entire atom.
But this is contextual, and not the size of the electron itself. I will call it the “contextual size”. Change the context — remove the electron from the atom, place it in a small metal box — and the distribution of the electron can grow or shrink. The proton, on the contrary, has a size inherent to it, about 100,000 times smaller than an atom. In no sense can you make a proton smaller than its inherent size without breaking it. In short, the contextual size cannot be less than the internal size. Having reduced the contextual size of an electron to a minimum, mainly through the scattering of high-energy electrons from other particles, we searched for their internal size. Nothing found yet.
So, we can say that experiments show that the inherent electron size is less than 10 -18 m. And how far the electron spreads in the form of a wave depends on the context.
About this property, you could not hear. It can conquer your brain (as I conquered!)
Among the strange properties of the quantum world there is a very strange fact (first discovered in the 1920s by Gaudsmit and Uhlenbeck , who tried to comprehend the data obtained from measurements of electrons in an atom) - elementary particles can spin without even having size! It’s impossible to imagine: at least it’s not available to me. Let's say it in a practical sense: electrons and many other particles of nature behave as if they are small spinning tops - if another object absorbs them, it makes this object spin a little. Imagine a spinning piece of soft clay falling onto a spinning table. Clay will stick to the table, and the table will begin to rotate.
Even more strange, each type of particle always rotates at the same speed! We say that for electrons the spin is 1/2; it is the smallest non-zero rotational speed that a particle is capable of possessing. We are also aware of other types of elementary particles with spins 1/2, 1, and (as we think) 0, and non-elementary particles with spins of 0, 1/2, 1, 3/2, 2, 5/2, and further , to very large values.
An electrically charged spinning ball would behave like a magnet, and you can guess that because electrons have charge and spin, they behave like magnets. And you are right! The fact that electrons behave like small magnets helps confirm the fact that they actually rotate. Ordinary, everyday magnets, made of, say, iron, acquire their magnetism from electrons; sets and sets of electrons, whose backs are neatly aligned, can create a large magnet from sets and sets of little ones!
Isn't it time to demonstrate the image of an electron in this article?
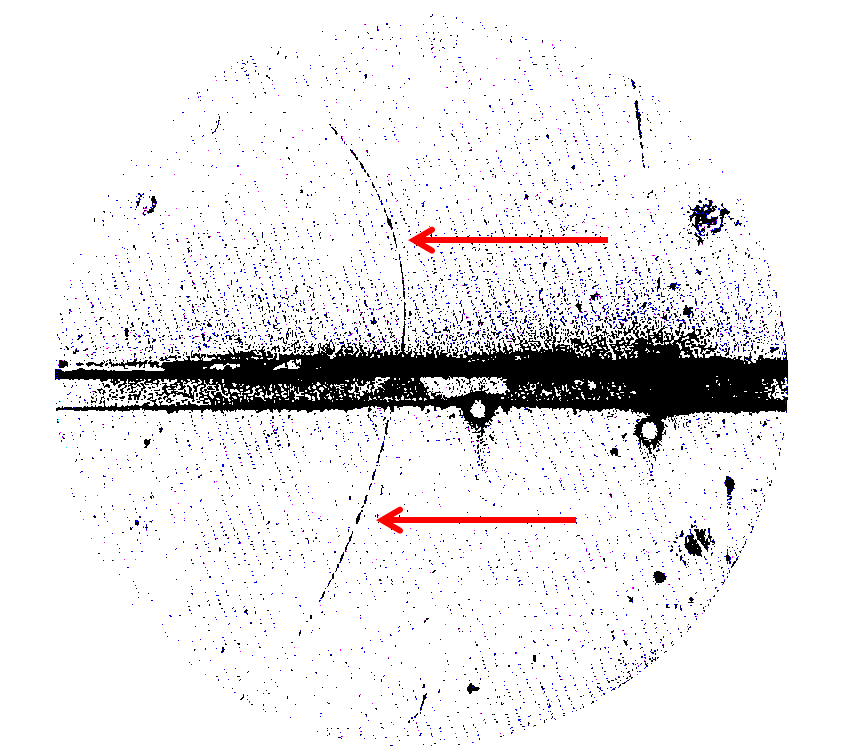
An electrically charged particle passes through a specially prepared bubble chamber , leaving behind a trace of bubbles. The bubbles quickly swell to visible size, and then this trace can be photographed. The magnetic field bends the path of the particles; the direction of the bend tells you whether the particle charge was positive or negative. This famous photo of 1933 demonstrates a thin curved path of bubbles, marked by red arrows, behaving in the same way as an electron track - except that the electron trace would have arched to the right. Bending in the wrong direction proves that the particle that left the trace has a positive charge, and therefore the trace is left by a positron, an antiparticle of the electron. Horizontal bar and diagonal lines are the artifacts of photography and experimental setup.
Unlike molecules and atoms, which are large enough to take photographs of them using special microscopes, it is impossible to make an electron image. It is simply too small and elusive. We can make images of traces of electrons passing through matter, as in the figure (it shows an anti-electron, a positron, but the electron would look almost exactly the same), but we cannot get images of electrons directly.
But our confidence in the existence of electrons is very strong, and our knowledge of their properties is very accurate. Where does this confidence come from?
This is an important question, because one of the most frequent questions asked to particle physicists is whether we actually know that these particles exist, or whether we are deceiving ourselves (and everyone else), and spending a lot of money on nonsense, which It turns out just hot air coming out of our heads.
Yes, we know what we are doing. And we know about this for over a hundred years. Part of our confidence is due to the above images. But there are many other sources of confidence, which I may write later.
By “elementary,” I mean that electrons are indivisible and not composed of smaller particles. With the help of “seemingly” I remind that they are elementary, as far as we are allowed to judge this by modern knowledge - what we know about electrons was obtained in experiments, and our experiments do not have infinite power. If the electrons are not elementary, but so small that our current experiments cannot break them down - they will look elementary in all the experiments we conducted in the past and present, but not in all future experiments. So someday - after all, 80 years ago, people believed that protons could be elementary, but they lacked knowledge, and 150 years ago, people thought that atoms could be elementary, but they lacked knowledge - we may find that electrons are not elementary. But for now, since all the experiments available to us demonstrate that they are elementary, we will conditionally assume that this is the case - remembering that this is partly an experimental fact, and partly - an assumption!
The electron became the first of the detected subatomic particles (the first object found, whose size was smaller than an atom). At the time of its discovery, in the 1890s (usually written in 1897, but this discovery was somewhat gradual), scientific debates about whether matter consists of atoms, or whether atoms were just fiction, convenient for describing the behavior of matter, coming to an end. But even those who believed in the existence of atoms did not necessarily believe that atoms were indivisible (as their name implied, derived from the Greek “uncut”). A generation later, by the mid-1930s, physicists confirmed the existence of atoms, understood their basic structure, and learned how to calculate their properties with high accuracy. They carried out these calculations using equations from the theory of the behavior of matter of the 1920s, called "quantum mechanics", which became necessary because the famous Newton equations could not cope with the description of the work of atoms. Many key checks on the accuracy of quantum mechanics have been associated with accurate measurements of the behavior of electrons inside and outside of atoms.
All electrons are identical and indistinguishable; if I change two of them in places, you cannot detect it. So I can write about the "property of the electron", and you can be sure that these properties are for all electrons. What properties are inherent in them?
')
Weight!
An electron has a mass - it is small compared to the mass of any atom, so you can usually forget about it in the elementary classes of chemistry, but it is not so small as to forget about it in particle physics and even in understanding the structure of atoms. Although electrons do not make a significant contribution to the mass of an atom, the mass of an electron is necessary to determine the size of an atom. This, in particular, is the importance of the field and the Higgs particle. This mass can be written in different ways, and each of the methods gives you a different perspective:
- It is equal to approximately 9 × 10 -31 kg = 0.000 000 000 000 000 000 000 000 000 9 kg.
- It is equal to about 0.05% (more precisely, 1/1838) of the mass of a hydrogen atom - the lightest atom in nature. Most of its mass is contained in its core.
- The energy stored in the electron mass, E = mc 2 , is equal to 0.000 511 GeV. This is 200,000 times more energy transferred by a single green photon. In particle physics, the particle mass is often written through the inverse relationship between energy and mass: for a stationary particle, m = E / c 2 . In these terms, the electron mass is 0.000511 GeV / c 2 .
Electric charge!
An electron has an electric charge - which means that it has electric and magnetic fields. An electrically charged force will act on an electrically charged particle in the presence of an electric field. It is these forces that keep electrons inside their atoms.
How big is the electric charge of an electron? Imagine static electricity - you walked along the carpet in your shoes, and then, touching the door handle, another person or computer (!!!), you will feel a spark. This spark transfers charge from one place to another - and usually it is 10 million million times the charge carried by an electron. Physicists measure charge using a randomly selected unit called pendant (just as time is measured in seconds and length in meters). A typical static electricity charge contains one millionth of a pendant. The magnitude of the electron charge is usually denoted by e, and e is approximately equal to 1.6 × 10 -19 C.
The size?
Electron size unknown; it may be a pointless object with no size, or it may have an extremely small size, the radius of which does not exceed 10 -18 m. It is at least 100,000,000 times smaller than the radius of an atom. Otherwise, we would see signs of electron size in experiments.
What does an electron actually look like? As I wrote in the article about atoms , it is difficult to define the concept of the size of an elementary particle, since an electron, although it is called a particle, is not some speck of dust or a grain of salt or sand. He also has wave properties. In an atom, electrons are in some sense distributed throughout the atom, as the sound wave propagates from the drum. In this sense, being inside an atom, they have the size of an entire atom.
But this is contextual, and not the size of the electron itself. I will call it the “contextual size”. Change the context — remove the electron from the atom, place it in a small metal box — and the distribution of the electron can grow or shrink. The proton, on the contrary, has a size inherent to it, about 100,000 times smaller than an atom. In no sense can you make a proton smaller than its inherent size without breaking it. In short, the contextual size cannot be less than the internal size. Having reduced the contextual size of an electron to a minimum, mainly through the scattering of high-energy electrons from other particles, we searched for their internal size. Nothing found yet.
So, we can say that experiments show that the inherent electron size is less than 10 -18 m. And how far the electron spreads in the form of a wave depends on the context.
Spin@
About this property, you could not hear. It can conquer your brain (as I conquered!)
Among the strange properties of the quantum world there is a very strange fact (first discovered in the 1920s by Gaudsmit and Uhlenbeck , who tried to comprehend the data obtained from measurements of electrons in an atom) - elementary particles can spin without even having size! It’s impossible to imagine: at least it’s not available to me. Let's say it in a practical sense: electrons and many other particles of nature behave as if they are small spinning tops - if another object absorbs them, it makes this object spin a little. Imagine a spinning piece of soft clay falling onto a spinning table. Clay will stick to the table, and the table will begin to rotate.
Even more strange, each type of particle always rotates at the same speed! We say that for electrons the spin is 1/2; it is the smallest non-zero rotational speed that a particle is capable of possessing. We are also aware of other types of elementary particles with spins 1/2, 1, and (as we think) 0, and non-elementary particles with spins of 0, 1/2, 1, 3/2, 2, 5/2, and further , to very large values.
Magnetism ↑
An electrically charged spinning ball would behave like a magnet, and you can guess that because electrons have charge and spin, they behave like magnets. And you are right! The fact that electrons behave like small magnets helps confirm the fact that they actually rotate. Ordinary, everyday magnets, made of, say, iron, acquire their magnetism from electrons; sets and sets of electrons, whose backs are neatly aligned, can create a large magnet from sets and sets of little ones!
Are you sure that electrons really exist?
Isn't it time to demonstrate the image of an electron in this article?

An electrically charged particle passes through a specially prepared bubble chamber , leaving behind a trace of bubbles. The bubbles quickly swell to visible size, and then this trace can be photographed. The magnetic field bends the path of the particles; the direction of the bend tells you whether the particle charge was positive or negative. This famous photo of 1933 demonstrates a thin curved path of bubbles, marked by red arrows, behaving in the same way as an electron track - except that the electron trace would have arched to the right. Bending in the wrong direction proves that the particle that left the trace has a positive charge, and therefore the trace is left by a positron, an antiparticle of the electron. Horizontal bar and diagonal lines are the artifacts of photography and experimental setup.
Unlike molecules and atoms, which are large enough to take photographs of them using special microscopes, it is impossible to make an electron image. It is simply too small and elusive. We can make images of traces of electrons passing through matter, as in the figure (it shows an anti-electron, a positron, but the electron would look almost exactly the same), but we cannot get images of electrons directly.
But our confidence in the existence of electrons is very strong, and our knowledge of their properties is very accurate. Where does this confidence come from?
This is an important question, because one of the most frequent questions asked to particle physicists is whether we actually know that these particles exist, or whether we are deceiving ourselves (and everyone else), and spending a lot of money on nonsense, which It turns out just hot air coming out of our heads.
Yes, we know what we are doing. And we know about this for over a hundred years. Part of our confidence is due to the above images. But there are many other sources of confidence, which I may write later.
Source: https://habr.com/ru/post/373943/
All Articles