Epigenetic clocks and other aging biomarkers
What is an aging biomarker and why is it needed?
We all know someone who is “perfectly preserved” for his age, and someone who is “old beyond his years”:

Actually, we need biomarkers of aging in order to be able to objectively say: yes, you are 50, but your health is at the level of 35 years. But you, a young man, should take a closer look at your health - your biological age is 10 years older than chronological , and this is fraught with a 48% increase in the risk of death.
What does aging and the likelihood of death? And despite the fact that in humans, as in most mammals, aging is accompanied by an exponential risk of mortality:
')

And if at 30 years old the annual probability of dying is 1 chance per thousand for you, then by 80 it rises 100 times. It is this age-related increase in the probability of death that gerontologists call aging. And no, aging is not “all living things”. There are species that, on the contrary, “younger” with age - their probability of death decreases, and fertility increases:

So, the goal of the fight against aging is to learn how to roll back the biological age to the level of today's healthy 25-year-old person, and fix it there. And the task of aging biomarker is to separate the biological age from the chronological (passport) age. That is, reliably show exactly where on this curve you are:
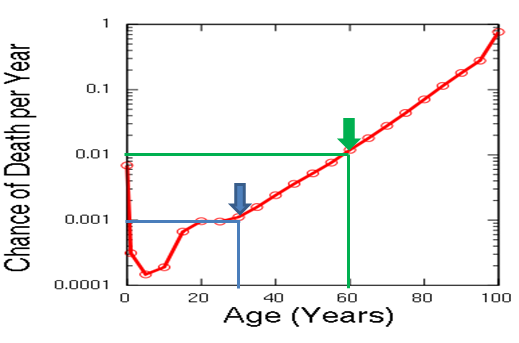
Therefore, a good biomarker should be highly correlated with mortality so that it can be used to determine the biological age. For example, if the biomarker shows that your current annual probability of death is 1/1000, then you are biologically 30, and if 1/100, then 60. Regardless of what your passport says. Because we die not according to our passport, but because of our health.
And of course, it is important for us to see the reverse dynamics of aging biomarkers: the body was “healed” with any proven rejuvenating therapy (for example, mice with the same fasting or rapamycin) and saw a decrease in biological age.
What is epigenetics?
Epi genetics is a “superstructure” above ( epi- = above ) genetics, a mechanism for controlling genes. Rather, there are several such mechanisms: methylation of the genes themselves, acetylation or methylation of the histones on which these genes are “wound”, and many other things that fall under the definition of epigenetic control.
Why do genes even need to be controlled? Firstly, because the organism's DNA is the same in all cell types, and different sets of genes must be active in the brain and skin cells. And also because different genes are responsible for different stages of development of the organism - the caterpillar and the butterfly have a very different profile of the activity of these same genes. As with us, in fact: in the womb, some genes are active, in childhood - others, in old age - third ones.
And as it turns out, with age the profile is on / off. different genes varies in all people almost equally. And what is even more interesting, it changes in a similar way in mice and other animals. That is, the epigenetic aging of the mouse is similar to the epigenetic aging of a person, only accelerated 40 times:
We observed tissue-specific age-related changes in DNA methylation [mice], the directional vector of which coincided with the observed changes in humans. These results further support the view that changes in DNA methylation are associated with chronological age and suggest that these processes are similar in different tissues, as well as between mammalian species.
At the same time, we even have common “gears” with mice in these hours of aging - the same genes that make up their composition :
Differentially methylated regions in mice are highly similar in nucleotide sequence to humans, and their methylation pattern is also very similar between the two species.
What is epigenetic clock?
In fact, “methylation clock” is just a certain set of on / off parameters that correlate best with age. How old is chronological or biological?
Initially, both with that and with another - after all at animals in wild conditions both chronological, and biological are almost identical. They do not drink or smoke, and do not eat at McDonalds. Therefore, the initial methylation hours are set (calibrated) according to the chronological age of each species, and then various ways to speed up or slow them down are tested to check whether the effects that prolong life at the same time slow down these hours, and those effects that shorten life, Does this watch speed up?
And yes! In smokers, diabetics, AIDS patients, or people with Down syndrome (who are aging much faster), the true biological age turned out to be higher than their chronological age. And in mice treated with various rejuvenating therapy, the biological age also decreased.
But more on this later. So far I will only mention that, in addition to humans, a high correlation between methylation hours and age has been established for a number of animals: rotifers , mice, chimpanzees , and even whales :

And what is so special about this very watch?
What is special about methylation hours is that they are highly correlated with mortality. For example, here in this large-scale study of Horvat , thirteen thousand people found that each year the clock was ahead of the methylation of chronological age (that is, if you are 45 and the clock is 46), there is a 2% to 4% increase in mortality risk.
Moreover, this observation worked in both directions and had a cumulative effect: for those with biological clocks overtaking age by 10 years, the risk of death increased by as much as 48% (1.04 10 = 1.48), and those who were “younger” by 5 years their age were 18% less likely to die.
Another study showed a high correlation between methylation hours and the risk of lung cancer in smokers. Moreover, both the risk and the coefficient of reliability of the correlation increased with age:
We also showed that the ability of the IEAA to predict lung cancer is highest among people aged 70 years and older. One unit increase in IEAA was associated with a 2.5-fold increase in lung cancer among the subgroup of people aged 70+, while for the entire 50+ year old cohort it increased the risk by only 50%.
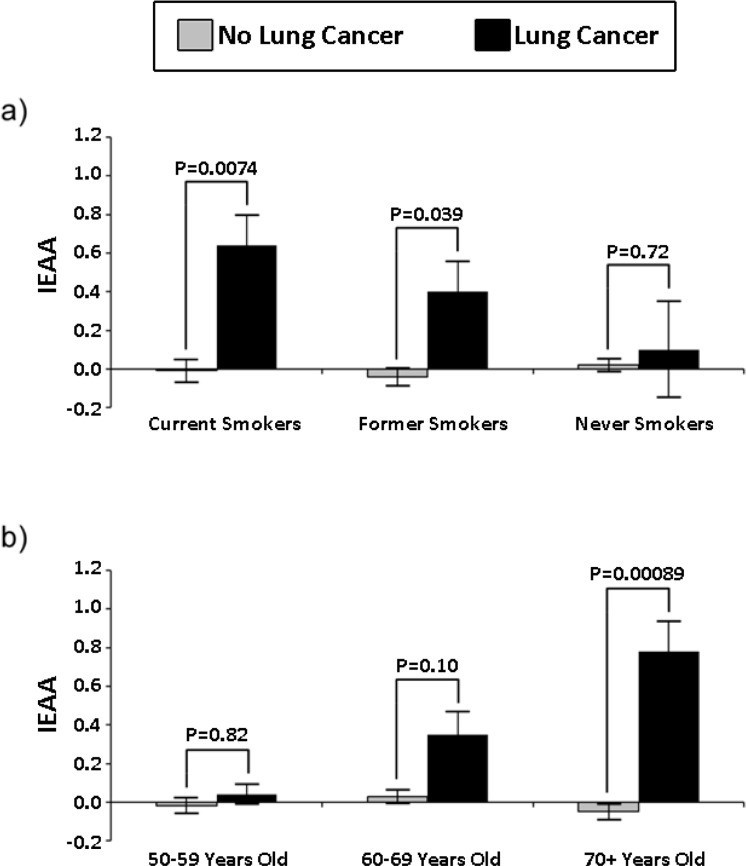
In this study , only 10 methylation sites were identified (for comparison, in the “Horvat clock” mentioned above, there were 353 such sites), the abnormal methylation of at least six of which increased the risk of mortality — from any cause or from cancer. or cardiovascular disease:

In this study, the Japanese showed generally deadly facts for the mitochondrial theory of aging: defects in mitochondrial respiration are not caused by the accumulation of breakdowns, but by epigenetic (programmed) changes. And with the epigenetic rollback of such old cells with the help of the Yamanaki factors, all defects of mitochondrial respiration disappear:
We reprogrammed human fibroblast lines, generating iPSC, and showed that reprogramming fibroblasts obtained from older people restores age-related defects in mitochondrial respiration. Therefore, these age-related phenotypes found in elderly fibroblasts are regulated reversibly and are similar to the differentiation phenotypes, since both are controlled by epigenetic regulation, and not by mutations in nuclear or mtDNA. Given that human aging can be viewed as a consequence of a programmed phenomenon, it is possible that epigenetic regulation also controls human aging.
The Japanese are talking. And the Japanese presented the greatest gift to humanity in the form of the very factors of Yamanaki. After all, they not only reset the epigenetic hours (both in humans and in mice ), but also significantly prolong the life of animals :
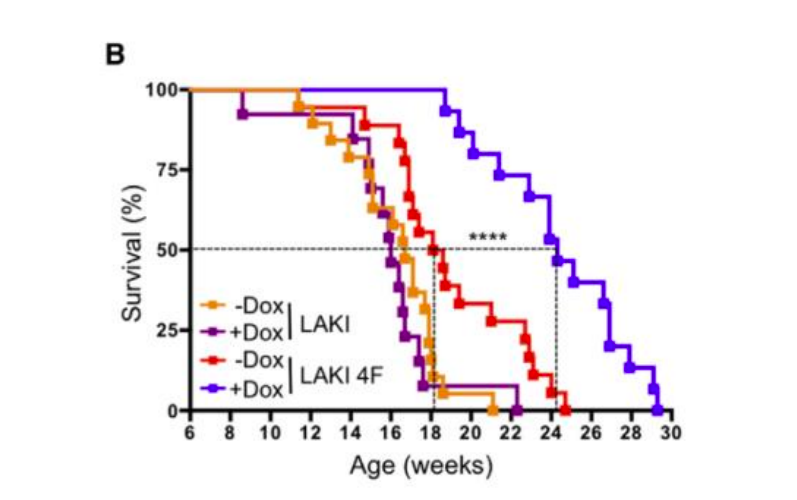
So how to get younger?
The main point of aging biomarkers is to look for the most effective ways to combat it with their help. Therefore, immediately after the methylation clock proved to be such a potential biomarker, scientists rushed to examine them to determine whether they reflect the effectiveness of various rejuvenating effects. And indeed, this relationship begins to manifest itself. Here is a summary of the last study already a couple of times mentioned Horvat, who is one of the leading experts in this area (blue arrows reduce methylation hours, and red arrows increase):
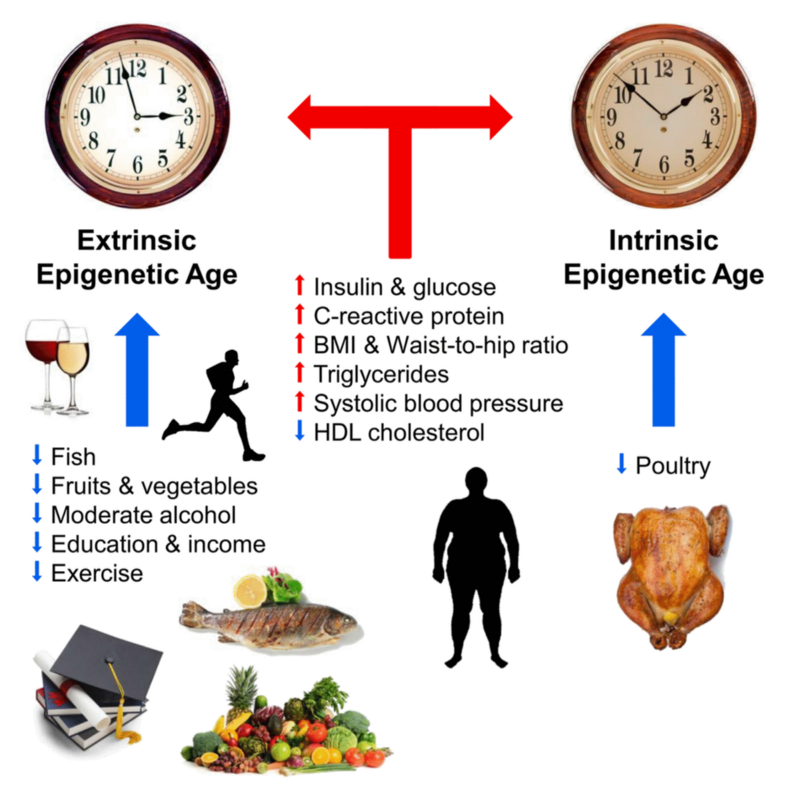
Much of the above is expected. A couple of surprises for me were that both moderate alcohol consumption and “good” cholesterol reduced methylation hours. Well, there will be a reason to drink for the health of Horvath.
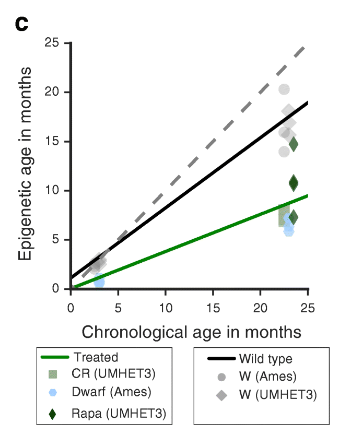
A much more targeted study on mice to evaluate the effects on hours of methylation of rapamycin, calorie restriction and genetic life-prolonging mutations was published not so long ago. And it also confirmed that all these interventions have a rejuvenating effect on methylation hours:
We formulated a model of epigenetic aging in mice and used it to find evidence that known life extension interventions slow down the epigenetic hours in the liver of mice.
By the way, the same researchers published a parallel article where they tried to identify exactly which regions of the genome are subject to age-related changes in methylation. After analyzing 42 million (!) Methylation sites, the authors concluded that the main targets for age-related changes are promoters and enhancers of highly expressed genes.
What confirms the hypothesis of programmed aging for me is that the key age change is a change in the expression profile of just a few key gene regulators at the top of the homeostasis control hierarchy, and this cascade entails all other age changes.
Vadim Gladyshev and his colleagues in their latest work also describe very similar findings that testify to several key genes:
The significance of various methylation sites was unevenly distributed in methylation hours. The sites formed several different clusters associated with the Hsf4, Kcns2, Map10, Tns2, Wnt3a, and Zscan2 genes. We found that 17 of the 18 CpG sites common to the subsets of methylation hours 1 and 2 were also present among the 90 CpG sites of the mDNAm watch. Most of these 17 CpG sites were located within Ciita, Cd200r4, Rasgef1c, Wnt3a and Zscan2 introns, and some were grouped.
The slowing down of methylation hours with the help of various anti-aging interventions was shown in this beautiful work :
It is important to note that we have found that biological interventions affect the hours of mice methylation, and therefore we assume that the prediction of hours reflects not only chronological, but also biological age.
I really liked this graph of age-related changes in the methylation level of 329 sites in different tissues:

It shows how some genes are activated with age, others - on the contrary, and still others remain unchanged.
By the way, the results of studies on twins confirm the correlation of methylation hours with mortality. The higher the biological age of one of the twins, the higher the likelihood that he will die first:
This hypothesis was confirmed by the classical analysis of survival, showing an increase in the risk of mortality by 35% (4-77%) for each 5-year-old age over the hours of methylation over chronological age. In addition, a twin analysis of twins revealed more than double the risk of mortality for a twin with a large age by methylation hours, as well as the dose-dependence of the probability of death, which increased by 3.2 (1.05-10.1) times for every 5 years the difference in age hours of methylation between twins, thereby demonstrating a stronger relationship of methylation hours with the probability of death in older people, taking into account family factors. In conclusion, our results confirm that methylation hours can be considered the biomarker of aging.
Summarizing this part, I can not say that I believe that in order to rejuvenate radically, you need to learn how to roll back the epigenetic clock directly. While we are just beginning to understand how to do it: thanks to the results of the Belmonte group, we have learned to knock lightly on this watch with a hammer in the form of Yamanaki factors. But ideally, I would like to find a key to our watch.
What other biomarkers are aging?
Among other biomarkers of aging, I want to mention locomotor activity, on the basis of which the Russian company Gero recently released a cool application that determines the biological age by the pattern of physical activity from your FitBit.
Also a good predictor of mortality is the thickness of the intima-media arterial complex. With regard to IGF-1, everything is difficult ( 1 , 2 , 3 , 4 , 5 , but 6 , 7 , 8 ), so I will deal with it in a separate post. Well, the grandfather of all biomarkers is the frailty index , over which gerontologists still beat the unequivocal translation: the “decrepitude index” sounds insulting, and the “fragility index” or “vulnerability index” does not fully convey the original meaning.
By the way, not so long ago I saw the news about a fresh article, which boldly claimed that the new version of this index developed by its authors reflects the biological age even better than “methylation hours”. At the very least, her headline was very ambitious: “ The frailty index outperforms ”.
But after reading it, I realized that the boldness of the statements of the authors is unfounded. Not only that their hypothesis, to put it mildly, is doubtful, but their very article their article disproves this hypothesis. To begin with, their new and improved index is a simple 34-item questionnaire. Which, at the same time, infarction and cystitis have an equal role:

And loud statements are not supported by their own data. Here is a table of the results of their analysis:
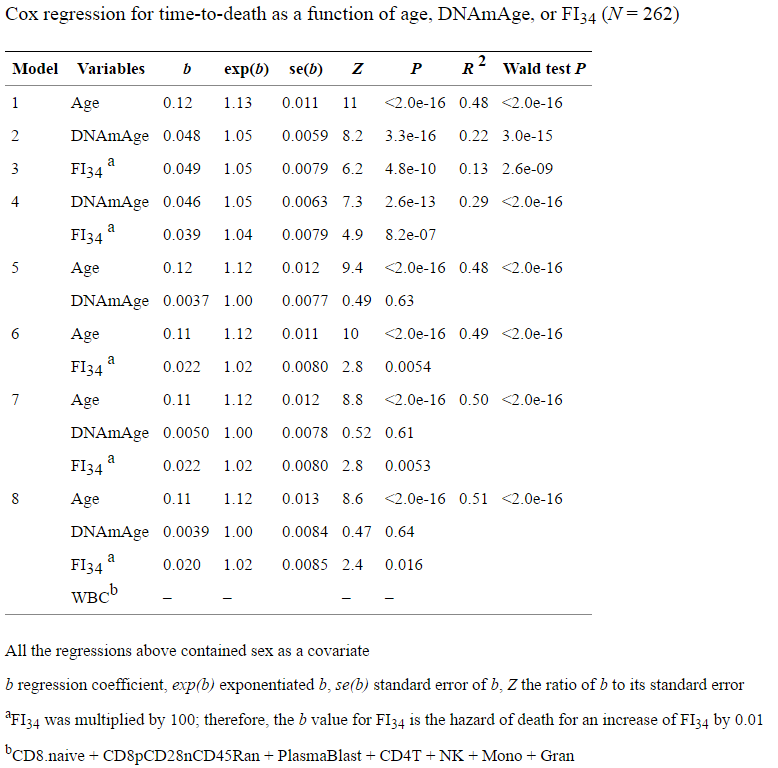
From it, we see that the regression by methylation hours (model No. 2) has a higher R 2 than the regression by their decrepitude index (model No. 3) - almost 2 times (0.22 versus 0.13). At the same time, the usual chronological age (model No. 1) has R 2 almost 4 times higher than their index, and 2 times higher than the methylation hours. I'm not even saying that R 2 is extremely low in all their models.
How do the authors substantiate their loud statements that their FI is better suited to the role of biological clocks than DNAmAge? But what about the lower p-value in model No. 7, and even limited in age to only eighty-year-olds:
In all Cox regression models, the chronological age was the best predictor of mortality across the entire cohort under study, which includes age categories from 60 to 103 (Fig. 3a). When Cox regression was limited to only 80-year-olds, FI34 was a better predictor of mortality than chronological age (P = 0.035 versus P = 0.054, respectively, Fig . 3b) . This indicates that FI34 is the best indicator of biological age in later years, when the accumulation of health deficit accelerates among the oldest.
That is, a lower p-value means better predictive power. Typical delusion of poorly understanding statistics:
Misconception # 13: Statistical significance is a property of the phenomenon being studied, and thus, statistical tests reveal significance.
Not! This misinterpretation is promoted when the researchers claim that they have found or have not found “evidence” of a statistically significant effect. The studied effect either exists or does not exist. "Statistical significance" is a dichotomous description of the value of P (which is lower than the chosen cut-off) and, therefore, is a property of the result of a statistical test, but it is not a property of the effect or the population under study.
Moreover, for their key conclusion, the authors took model No. 7 as the basis, where both considered parameters (FI and DNAmAge) are presented simultaneously, and also together with the chronological age with which they both correlate (that is, most likely they are not independent, disrupting one of the regression conditions). Yes, and narrowed the sample only to 80-year-olds - that is, to a very narrow segment of that parameter (chronological age), which best explains the variation of the entire data array (since the model number 1 has R 2 several times higher than other models), and then We are happy to report that the age on such a narrow age segment has a low p-value.
About the fact that the model of their index of decrepitude of R 2 is almost 2 times lower than the model of DNAmAge, I have already mentioned (0.13 versus 0.22). And the fact that adding additional parameters to the existing chronological age is meaningless is evident from the fact that R 2 of such models (0.48–0.50) is almost identical to the original (0.48 model No. 1).
Well, in conclusion, it is worth noting that the value of the very concept of decrepitude index proposed by the authors is close to zero. The biomarker is valuable in that it can change in both directions, because aging therapy is designed to reduce the biological age. And the proposed decrepitude index is mainly based on historical parameters (did you have a stroke / heart attack / etc.). Therefore, even if aging therapy rejuvenates your heart, brain and kidneys, this will hardly affect the historical index of decrepitude. And on the methylation clock or locomotive - completely.
So congratulations to all of us with new biomarkers of aging, and wish us happiness, health, and a very long life!

Source: https://habr.com/ru/post/373575/
All Articles