Atoms: building blocks of molecules
If molecules — the basic structures involved in chemistry — are the words that make up all the materials around us, then atoms are letters, the building blocks of molecules. Words come in different lengths, and a typical molecule can also contain several atoms, or several hundred, or even one hundred thousand atoms. The molecule of table salt NaCl consists of two atoms, sodium Na and chlorine Cl. The water molecule H 2 O contains two hydrogen atoms and one oxygen. The table sugar molecule C 12 H 22 O 11 contains 12 carbon atoms, 11 oxygen and 22 hydrogen, organized in a certain way.
How do we know about the existence of atoms? Sometimes they can be "seen", just as we see the molecules that they can form. Not with eyes, but more advanced devices. One of the methods uses a scanning tunneling microscope capable of showing atoms in a crystal or even moving them one at a time. Another method uses our ability to trap ions (slightly modified atoms — see below for details).
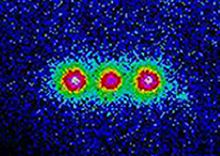
In the photo - three ions caught simultaneously. Light falls on them, they absorb it and emit it again. Re-emitted light can be detected, so we can see where the ions are - something like the reflection of light from a small but bright diamond can help us find it.
How many types of atoms exist? Types are called "chemical elements" and their exact number depends on how to count them. But suppose that the atomic alphabet consists of about a hundred chemical elements, and we will return to the subtleties of calculation later. Just as we could assign letters from A to Z to numbers from 1 to 33, each element is assigned not only a name, but also an atomic number (denoted by "Z"). The simplest atoms are hydrogen, their atomic number is 1. The most complex atoms are found in abundance in nature, this is uranium with atomic number 92. Others are oxygen (8), nitrogen (7), calcium (20), krypton (36), lanthanum (57), platinum (78). For a complete list, look at the periodic table of periodic elements . Each element has its own chemistry - the way it behaves inside molecules - approximately the way each letter has its own rules according to which it can occur in words.
')
Questions you can ask about atoms:
1. What are atoms made of?
2. What is the meaning of the atomic number?
3. What is the main source of differences in the chemical behavior of atoms of different elements?
4. To what extent are the different atoms of one element similar to each other?
5. How are parts of an atom held together?
6. Why are atoms held together and form molecules?
It turns out that all these questions are best answered by starting with the first: what are the atoms made of? Atoms consist of what is commonly called "subatomic particles" (unfortunately, this term is incorrect, since these "particles" have certain properties that are not inherent in particles). More specifically, atoms consist of a set of small and very light electrons surrounding a tiny, but heavy atomic nucleus, which contains most of the mass of the atom. The core consists of other "particles", in turn, also consisting of other "particles", and we still get to them.
Often we see images of atoms drawn on chemistry books, on commercials and warning signs. An example is rice. 1. He conveys a very rough idea of how an atom works: outside it has a certain number of electrons (blue), and they rotate around the central atomic nucleus. The nucleus is a cluster of protons (red) and neutrons (white).

Fig. one
Now we can answer the 2nd question: what does the atomic number Z mean? It is simply the number of protons in the nucleus. Oxygen has atomic number 8, and it has 8 protons in the nucleus.
Under the simplest conditions, the atomic number also equals the number of electrons of an atom. With the number of neutrons getting harder, we'll come back to this later. Electrons have a negative electric charge (-e), and protons have a positive (+ e). Neutrons are neutral, they have no electric charge. When the number of electrons and protons coincides, their charges are mutually destroyed, and the atom of an electric charge is not observed - such an atom is neutral.
But there is nothing unusual — for example, in the process of the formation of molecules — if an atom acquires or loses one or more external, valence electrons. In this case, the electric charges of electrons and protons are not destroyed, and the resulting charged atom is called an ion.
Although rice. 1 roughly describes the architecture of the atom - electrons are really outside, and the nucleus, consisting of protons and neutrons, is in the middle - it does not convey the real form and essence of the atom, because it is not made to scale, but we live in a quantum world in which objects behave in such a way that they are difficult to draw or imagine.
The scale problem can be sorted out by drawing a more accurate (although still imperfect) image, fig. 2

Figure 2. Atom - mostly empty (gray area). Electrons move rapidly along it (blue dots, drawn not to scale, but much more). In the center there is a heavy core (red and white dots, drawn more than in scale).
This is what I tried to convey with this image. First, the electrons are very, very small, so small that we could not measure their size - it may be that they are point and have no size, but they are definitely not more than 1/100 000 000 of the diameter of an atom. Secondly, the nuclei (and protons with neutrons, their components) are also extremely small, although they are larger than electrons. Their size is measured, and it is about 10,000 - 100,000 times smaller than the diameter of an atom. Atom is a bit like a village. The protons and neutrons in the nucleus are large houses located in the center of the village, and the electrons are far from scattered farm houses. Grain crops grow in most of the countryside and there are no houses. And although the territory, which is considered part of the village, may be large, the area actually occupied by the houses is very small.
But this analogy is not complete, since electrons, unlike farm houses, very quickly move through the gray region in the picture and around the nucleus with speeds of the order of 1% of the speed of light. The territory covered by them is usually not spherical, but of a more complex form, moreover, not all electrons move along the same territory.
But, as I warned you, rice. 2 is also not accurate. First, it would be necessary to draw a nucleus a thousand times smaller, and electrons — a million times smaller, only then they would not be visible. If an atom were the size of your bedroom, then its core would be the size of a speck of dust. Compared with their components, atoms are huge! In a sense, most of the atom is emptiness!
Secondly, the image does not convey the turbid nature of quantum mechanics. The equations of quantum mechanics describe and predict the behavior of molecules, atoms and subatomic particles, and these equations tell us that these particles can have very strange and non-intuitive properties. Although electrons are in some sense point (for example, if you want to push two electrons together, you will find that you can move them together at an arbitrarily small distance, and they will not give out their internal structure, if it exists at all) it is possible to make it so that if they are left alone, they will spread like a wave and fill the entire gray space in fig. 2. If it sounds strange, it’s not because you didn’t understand something: it’s strange and hard to think about. I don’t really know how to draw an atom so as not to mislead you, and experts still argue about how to think about it best. So for now just accept it as a strange fact.
The size of the electron is too small to measure, and its mass is so small that the electron can spread throughout the atom. But the nucleus has a completely measured and known size, and its mass is so large - more than 99.9% of the mass of the entire atom - that it is not distributed at all in space. The core sits in the middle of the gray area.
The best way to describe an atom comes to my mind: most of the mass of an atom is contained in the nucleus located in its center, around which extremely small electrons of much smaller mass were distributed, and this was done completely differently from how the particles behave, filling the entire gray area of fig. 2
The small size of the nucleus relative to the full size of the atom, and the fact that it is usually located in its center, explains why it plays a relatively weak role in chemistry. Chemistry occurs — that is, molecules form and change — when atoms approach each other, and this happens when the outer, valence electrons of one atom closely approach the outer electrons of another — when the edge of the gray region of one atom approaches the edge of the gray region of another. In chemical processes, the atomic nucleus remains in the centers of the atoms, and never approaches other nuclei. The main role of the nucleus is to provide a positive charge that holds electrons and most of the mass (which determines how difficult it is for other objects to move this atom).
This answers the third question: the chemistry of the atom is mainly determined by the details related to its external electrons. These details can be learned (in a complicated way, through the equations of quantum mechanics), based on the atomic number Z.
Instead of taking up chemistry, a topic that will be enough for a whole course, we will go one level down to subatomic particles, answering other questions along the way. We list the issues that we have dealt with, and questions that have yet to be studied.
1. What are atoms made of? Outside - electrons, in the center - the atomic nucleus (of protons and neutrons).
2. What is the meaning of the atomic number? This is the number of protons in the nucleus of an atom, which, under normal conditions, is equal to the number of electrons surrounding it.
3. What is the main source of differences in the chemical behavior of atoms of different elements? The properties of external electrons determined by the total number of electrons of each element, for example, atomic number.
4. To what extent are the different atoms of one element similar to each other? We will discuss this in the article on isotopes.
5. How are parts of an atom held together? We will discuss this in an article on the role of electrical forces and quantum mechanics.
6. Why are atoms held together and form molecules? We will discuss this in an article on the role of electrons and electrical forces in the construction of molecules from atoms.
And here's another question that could arise when studying rice. 2:
If the atom is mostly empty, why do objects appear to be solid? Why not reach out through the computer screen if the screen consists of atoms, mostly empty?
How do we know about the existence of atoms? Sometimes they can be "seen", just as we see the molecules that they can form. Not with eyes, but more advanced devices. One of the methods uses a scanning tunneling microscope capable of showing atoms in a crystal or even moving them one at a time. Another method uses our ability to trap ions (slightly modified atoms — see below for details).

In the photo - three ions caught simultaneously. Light falls on them, they absorb it and emit it again. Re-emitted light can be detected, so we can see where the ions are - something like the reflection of light from a small but bright diamond can help us find it.
How many types of atoms exist? Types are called "chemical elements" and their exact number depends on how to count them. But suppose that the atomic alphabet consists of about a hundred chemical elements, and we will return to the subtleties of calculation later. Just as we could assign letters from A to Z to numbers from 1 to 33, each element is assigned not only a name, but also an atomic number (denoted by "Z"). The simplest atoms are hydrogen, their atomic number is 1. The most complex atoms are found in abundance in nature, this is uranium with atomic number 92. Others are oxygen (8), nitrogen (7), calcium (20), krypton (36), lanthanum (57), platinum (78). For a complete list, look at the periodic table of periodic elements . Each element has its own chemistry - the way it behaves inside molecules - approximately the way each letter has its own rules according to which it can occur in words.
')
Questions you can ask about atoms:
1. What are atoms made of?
2. What is the meaning of the atomic number?
3. What is the main source of differences in the chemical behavior of atoms of different elements?
4. To what extent are the different atoms of one element similar to each other?
5. How are parts of an atom held together?
6. Why are atoms held together and form molecules?
It turns out that all these questions are best answered by starting with the first: what are the atoms made of? Atoms consist of what is commonly called "subatomic particles" (unfortunately, this term is incorrect, since these "particles" have certain properties that are not inherent in particles). More specifically, atoms consist of a set of small and very light electrons surrounding a tiny, but heavy atomic nucleus, which contains most of the mass of the atom. The core consists of other "particles", in turn, also consisting of other "particles", and we still get to them.
Drawn atom
Often we see images of atoms drawn on chemistry books, on commercials and warning signs. An example is rice. 1. He conveys a very rough idea of how an atom works: outside it has a certain number of electrons (blue), and they rotate around the central atomic nucleus. The nucleus is a cluster of protons (red) and neutrons (white).

Fig. one
Now we can answer the 2nd question: what does the atomic number Z mean? It is simply the number of protons in the nucleus. Oxygen has atomic number 8, and it has 8 protons in the nucleus.
Under the simplest conditions, the atomic number also equals the number of electrons of an atom. With the number of neutrons getting harder, we'll come back to this later. Electrons have a negative electric charge (-e), and protons have a positive (+ e). Neutrons are neutral, they have no electric charge. When the number of electrons and protons coincides, their charges are mutually destroyed, and the atom of an electric charge is not observed - such an atom is neutral.
But there is nothing unusual — for example, in the process of the formation of molecules — if an atom acquires or loses one or more external, valence electrons. In this case, the electric charges of electrons and protons are not destroyed, and the resulting charged atom is called an ion.
More realistic atom
Although rice. 1 roughly describes the architecture of the atom - electrons are really outside, and the nucleus, consisting of protons and neutrons, is in the middle - it does not convey the real form and essence of the atom, because it is not made to scale, but we live in a quantum world in which objects behave in such a way that they are difficult to draw or imagine.
The scale problem can be sorted out by drawing a more accurate (although still imperfect) image, fig. 2

Figure 2. Atom - mostly empty (gray area). Electrons move rapidly along it (blue dots, drawn not to scale, but much more). In the center there is a heavy core (red and white dots, drawn more than in scale).
This is what I tried to convey with this image. First, the electrons are very, very small, so small that we could not measure their size - it may be that they are point and have no size, but they are definitely not more than 1/100 000 000 of the diameter of an atom. Secondly, the nuclei (and protons with neutrons, their components) are also extremely small, although they are larger than electrons. Their size is measured, and it is about 10,000 - 100,000 times smaller than the diameter of an atom. Atom is a bit like a village. The protons and neutrons in the nucleus are large houses located in the center of the village, and the electrons are far from scattered farm houses. Grain crops grow in most of the countryside and there are no houses. And although the territory, which is considered part of the village, may be large, the area actually occupied by the houses is very small.
But this analogy is not complete, since electrons, unlike farm houses, very quickly move through the gray region in the picture and around the nucleus with speeds of the order of 1% of the speed of light. The territory covered by them is usually not spherical, but of a more complex form, moreover, not all electrons move along the same territory.
But, as I warned you, rice. 2 is also not accurate. First, it would be necessary to draw a nucleus a thousand times smaller, and electrons — a million times smaller, only then they would not be visible. If an atom were the size of your bedroom, then its core would be the size of a speck of dust. Compared with their components, atoms are huge! In a sense, most of the atom is emptiness!
Secondly, the image does not convey the turbid nature of quantum mechanics. The equations of quantum mechanics describe and predict the behavior of molecules, atoms and subatomic particles, and these equations tell us that these particles can have very strange and non-intuitive properties. Although electrons are in some sense point (for example, if you want to push two electrons together, you will find that you can move them together at an arbitrarily small distance, and they will not give out their internal structure, if it exists at all) it is possible to make it so that if they are left alone, they will spread like a wave and fill the entire gray space in fig. 2. If it sounds strange, it’s not because you didn’t understand something: it’s strange and hard to think about. I don’t really know how to draw an atom so as not to mislead you, and experts still argue about how to think about it best. So for now just accept it as a strange fact.
The size of the electron is too small to measure, and its mass is so small that the electron can spread throughout the atom. But the nucleus has a completely measured and known size, and its mass is so large - more than 99.9% of the mass of the entire atom - that it is not distributed at all in space. The core sits in the middle of the gray area.
Atom and its chemistry
The best way to describe an atom comes to my mind: most of the mass of an atom is contained in the nucleus located in its center, around which extremely small electrons of much smaller mass were distributed, and this was done completely differently from how the particles behave, filling the entire gray area of fig. 2
The small size of the nucleus relative to the full size of the atom, and the fact that it is usually located in its center, explains why it plays a relatively weak role in chemistry. Chemistry occurs — that is, molecules form and change — when atoms approach each other, and this happens when the outer, valence electrons of one atom closely approach the outer electrons of another — when the edge of the gray region of one atom approaches the edge of the gray region of another. In chemical processes, the atomic nucleus remains in the centers of the atoms, and never approaches other nuclei. The main role of the nucleus is to provide a positive charge that holds electrons and most of the mass (which determines how difficult it is for other objects to move this atom).
This answers the third question: the chemistry of the atom is mainly determined by the details related to its external electrons. These details can be learned (in a complicated way, through the equations of quantum mechanics), based on the atomic number Z.
Instead of taking up chemistry, a topic that will be enough for a whole course, we will go one level down to subatomic particles, answering other questions along the way. We list the issues that we have dealt with, and questions that have yet to be studied.
1. What are atoms made of? Outside - electrons, in the center - the atomic nucleus (of protons and neutrons).
2. What is the meaning of the atomic number? This is the number of protons in the nucleus of an atom, which, under normal conditions, is equal to the number of electrons surrounding it.
3. What is the main source of differences in the chemical behavior of atoms of different elements? The properties of external electrons determined by the total number of electrons of each element, for example, atomic number.
4. To what extent are the different atoms of one element similar to each other? We will discuss this in the article on isotopes.
5. How are parts of an atom held together? We will discuss this in an article on the role of electrical forces and quantum mechanics.
6. Why are atoms held together and form molecules? We will discuss this in an article on the role of electrons and electrical forces in the construction of molecules from atoms.
And here's another question that could arise when studying rice. 2:
If the atom is mostly empty, why do objects appear to be solid? Why not reach out through the computer screen if the screen consists of atoms, mostly empty?
Source: https://habr.com/ru/post/373435/
All Articles