Nuclear death of stars
In the end, it took the Universe ten billion years to evolve before life became possible. The evolution of stars and new chemical elements in nuclear furnaces of stars were irreplaceable preconditions for the emergence of life.
- John Palkinhorn
The number of atoms in your body is huge - about 10 28 . Half of them - hydrogen atoms, and all the rest - from lithium to uranium - originated inside the stars, and were thrown into the Universe, after which, after billions of years, gathered inside you.
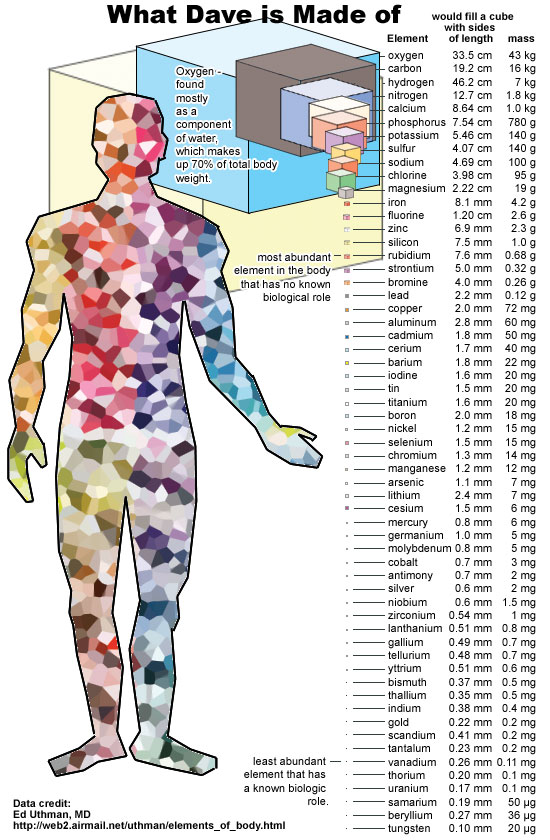
And a large number of these atoms did not come out of nowhere, but specifically from the supernova! Our story begins when the earliest elements in the universe, hydrogen and helium, come together in massive lumps due to the irresistible force of gravity, and form the first stars.

The scatter of masses of emerging stars is huge. A few percent will look like our Sun, a star of the G-class, but most will be of lesser mass, colder and redder. Approximately 90-95% of stars belong to the coldest classes, K- and M-.

But regardless of the class, the star receives energy from nuclear reactions. It takes the nucleus of four hydrogen atoms (just protons) and synthesizes from them a helium nucleus containing two protons and two neutrons.
')
The most intelligent in this place will begin to object, and quite rightly.

Neutrons are heavier than protons, so how can you turn four protons into two protons and two neutrons?
But we are not creating two free protons and two free neutrons, we are creating one bound helium nucleus. And if four protons are placed on one side of the balance, and a helium nucleus on the other, we find that protons are about 0.7% heavier than the nucleus.
Over time, while the temperature inside the star is high enough and there is enough hydrogen for helium synthesis, it burns its nuclear fuel. And this difference in 0.7% of the mass between hydrogen and helium - it is emitted in the form of energy, thanks to our old friend E = mc 2 .
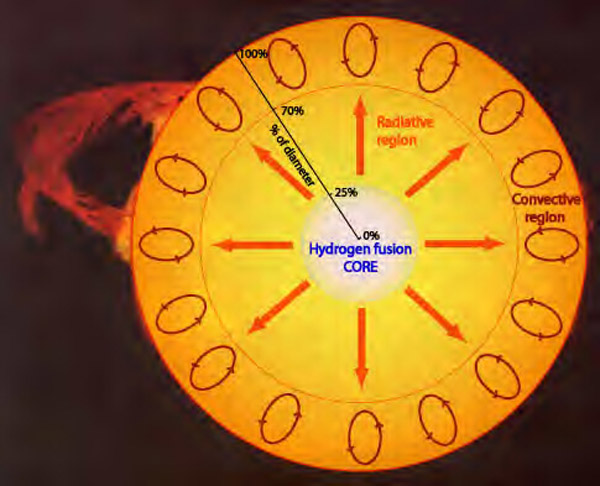
And all the stars, from supermassive and rare stars of class O (they exist less than 0.1% of all stars!), To numerous small stars of class M, burn hydrogen and turn it into helium.
But not everyone does it at the same speed. The oldest stars of class M have still not completed the conversion of hydrogen to helium, and a star of class O can burn all the hydrogen in the core in a million years. With the exception of M-class stars, which will never warm up to the required high temperatures, all other stars, including our Sun several billion years later in the future, will swell up to a red giant burning hydrogen in a shell around the helium core.
After some time, the temperature, pressure and density in the helium nucleus increase to such an extent that synthesis begins, turning every three helium atoms into a carbon atom.

Carbon is more stable than helium; therefore, as a result, even more energy is released through E = mc 2 . For class K stars, this is the final stop. When helium in the core ends, the outer layers explode around the area, and a planetary nebula appears, and the core shrinks with time, which leads to the appearance of a white dwarf with a mass comparable to the Sun and the size of the Earth.

More massive stars continue to burn heavier atoms and receive oxygen and neon, their inner layers synthesize ever heavier elements. But elements with large atomic numbers are very difficult to synthesize. Even bright and massive blue stars of class A can synthesize only silicon and sulfur, the fourteenth and sixteenth elements (and the stars of class A are only 1%!). You can even be a heavy and less bright star of class B, but the fate of such a star is all the same to discard the outer layers, creating a planetary nebula, and leave behind a white dwarf the size of Earth and a mass approximately equal to the sun.

But approximately one of the 800 stars appearing in the Universe turns out to be massive enough to go beyond silicon and create all the heavy elements up to the limits of what is possible in the stars: iron, nickel and cobalt.
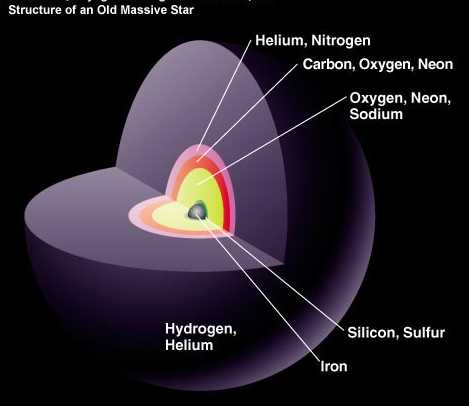
It would be wrong to call them "old" massive stars - their age can be 1% of the current age of the Sun, which still burns hydrogen in the core! But when the cores of massive supergiants increase quite strongly, then (predominantly) the iron atoms inside them face a problem.

So far, we have synthesized heavy elements from the lungs, and emitted energy with each successful step. But now the pressure on the iron atoms in the core is very large, but they have nowhere to go to release energy. Unless, of course, they collapse.
And with stars of approximately eight solar masses, this is exactly what happens. There is a threshold mass of the nucleus - about 1.38 solar masses - and if it is exceeded, then the iron atoms in the core collapse. The destruction of such a number of atoms - 10 56 - releases a huge amount of energy at once! While the core collapses to a neutron star (mass with the Sun and the size of a small asteroid) or a black hole, the outer layers receive a charge of energy comparable to the first seconds of the Big Bang.

The release of energy not only scatters the outer layers of the star around the light years, but also makes possible the appearance of all known elements of the Periodic Table. It creates not only uranium, but also heavier elements - plutonium, curium, and even heavier and short-lived elements. The reason why uranium and plutonium are the hardest elements of the natural conditions on Earth is that the heavier atoms had enough time for decay.

So, when we see a supernova, we witness the formation of all the elements heavier than iron found on Earth. And these elements do not appear anywhere else in the Universe!
But if a star is not born one of the 800 stars that have enough mass to create a type II supernova, this does not mean that it will never become a supernova. On the contrary, the stars that burned all the fuel and shrunk to white dwarfs get a second chance!

White dwarfs can capture matter from a companion star, as in the illustration above, or combine with another white dwarf, as in the video below.
In any case, when the total mass pressing on the atoms of the white dwarf exceeds the limit that they can withstand, the core of a dead star collapses. But this time it consists not of iron, but of carbon. In less than a second, the temperature exceeds the carbon needed to start the reaction, and after that an uncontrolled synthesis reaction occurs!
This is another type of supernova, type Ia. In this case, the synthesis reaction destroys the whole white dwarf, after which there is neither a neutron star nor a black hole - nothing at all!
This is exactly what happened to this star in the Pinwheel galaxy (Messier 101) 21 million years ago!

It is in the death of stars that all elements of the Universe are created, except for hydrogen and helium. In addition, all elements heavier than iron, including silver, gold, iodine, mercury, tin, lead, uranium, are created by supernovae. As Karl Sagan said,
We are made up of stellar material that has taken its fate into its own hands.
And we are not just star material, but supernova material! This is our common story, causing admiration and humility at the same time. Generations of stars lived, died, and re-used created elements to make the Earth appear after billions of years. This story is happening now, in countless distant galaxies throughout the universe.
Source: https://habr.com/ru/post/372711/
All Articles