How do lithium-ion batteries explode
Recently, the topic of spontaneous combustion of lithium-ion batteries often flashes in the news headlines: either the smartphone lights up, the hoverboard, or the car. So what happens inside the battery during thermal acceleration and why does spontaneous combustion occur?

Lithium-ion batteries consist of an anode and a cathode, separated by a porous polymer separator. The cathode active material is most often transition metal oxides with lithium ions embedded in the crystal. Graphite is commonly used in the anode. The electrolyte, which is filled with an electrochemical cell, is an organic solution of lithium salts. When the manufacturer first charges, when lithium is embedded in the anode on the electrodes (especially on the anode), a protective ion-conducting layer (SEI) is formed, consisting of decomposed electrolyte. This layer protects the electrodes from parasitic reactions with electrolyte.

')
The most common cause of spontaneous combustion of batteries is a short circuit inside the electrochemical cell. Electrical contact between the anode and the cathode can occur for many reasons. This may be, for example, mechanical damage to the cell. Another internal short circuit arises due to the violation of production technology in case of uneven cutting of electrodes or metal particles between the anode and cathode, which leads to damage to the porous separator. Also, the cause of the internal short circuit may be the "germination" of metal lithium chains (dendrites) through the separator. This effect occurs if lithium ions do not have time to integrate into the anode crystal when charging too fast or at low temperatures, as well as if the cathode active material capacity exceeds the anode capacity, as a result microscopic deposits appear on the anode surface, which gradually grow.
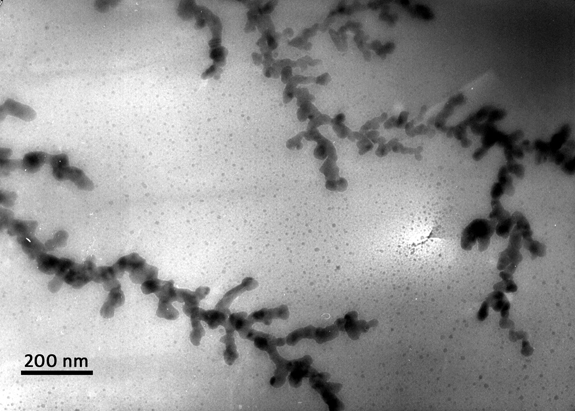
So, after a short circuit occurs, the battery starts to heat up. When the temperature reaches 70-90 ° C, the ion-conducting protective layer on the anode begins to decompose. And then lithium, built into the anode, reacts with electrolyte, releasing volatile hydrocarbons: ethane, methane, ethylene, etc. But, despite the presence of such an explosive mixture, ignition does not occur, since there is no oxygen in the system yet.
Since the reaction with the electrolyte is exothermic, the temperature and pressure inside the battery continue to rise. When the temperature reaches 180–200 ° C, the cathode material, usually a transition metal oxide with lithium embedded in the crystal, reacts to disproportionation and releases oxygen. This is where the spontaneous combustion takes place and an even sharper temperature jump. In parallel, there is a thermal decomposition of the electrolyte (200-300 ° C), which also produces heat. It looks like this:
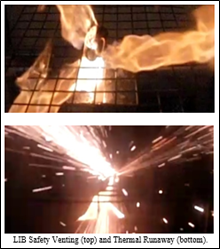
And, in the end, graphite enters into reaction with the electrolyte (if it still remains), and when the temperature reaches 660 ° C, the aluminum current collector melts. Above 900 ° C, the temperature usually does not rise, since there is nothing to decompose.
In addition to internal short circuits, there are other causes of spontaneous combustion: overheating of the battery, improper charging / discharging (exceeding the maximum allowable voltage, charging at high currents, too deep discharge), etc. But all these reasons lead to one result: thermal acceleration and decomposition of the electrolyte when interacting with the electrodes. Only the order of the above reactions and their speed differ.
Naturally, battery manufacturers have provided self-ignition protection systems, and the larger and more powerful the battery, the more protection it contains. One type of protection against a small short circuit is a porous separator, which, with a local increase in temperature, becomes impenetrable and prevents, for example, the further growth of dendrites inside the battery. But sometimes the temperature rises too quickly and the separator simply melts, causing the anode to come into contact with the cathode.
The batteries are also equipped with fuses and valves, which, when the pressure and temperature inside increase, either disconnect the electrodes from the circuit, or contribute to the release of accumulated gas. In the latter case, since the gases are flammable, when in contact with oxygen there is a flame outside. An example of the action of safety valves could be observed in an accident involving the Tesla car Model S, where the battery was punctured by a large metal object. Since in Tesla, the battery valves were aimed down on the asphalt and the individual blocks were well isolated from each other, only the front of the battery burned (as Elon Musk said, if the same metal object broke through the gasoline tank, the car would burn completely).

By the way, the thermal insulation of individual units in a large battery is very important. If, in the above example, the Tesla battery did not catch fire completely due to good thermal insulation, in the case of a battery on board the Boeing 787, spontaneous combustion occurred because the blocks were not sufficiently isolated from each other, which led to overheating of the entire system.

Also, lithium-ion batteries are equipped with controllers, sensors, charge balancers, etc. You can read more about battery safety systems here .
As can be seen from this post, the most dangerous component of a battery is an electrolyte, which decomposes into flammable components with increasing temperature. Today scientists are trying to find more stable alternatives: ionic liquids, polymer electrolytes, solid ceramic electrolytes, etc. But this is a separate topic ...
Sources:

Lithium-ion batteries consist of an anode and a cathode, separated by a porous polymer separator. The cathode active material is most often transition metal oxides with lithium ions embedded in the crystal. Graphite is commonly used in the anode. The electrolyte, which is filled with an electrochemical cell, is an organic solution of lithium salts. When the manufacturer first charges, when lithium is embedded in the anode on the electrodes (especially on the anode), a protective ion-conducting layer (SEI) is formed, consisting of decomposed electrolyte. This layer protects the electrodes from parasitic reactions with electrolyte.

')
The most common cause of spontaneous combustion of batteries is a short circuit inside the electrochemical cell. Electrical contact between the anode and the cathode can occur for many reasons. This may be, for example, mechanical damage to the cell. Another internal short circuit arises due to the violation of production technology in case of uneven cutting of electrodes or metal particles between the anode and cathode, which leads to damage to the porous separator. Also, the cause of the internal short circuit may be the "germination" of metal lithium chains (dendrites) through the separator. This effect occurs if lithium ions do not have time to integrate into the anode crystal when charging too fast or at low temperatures, as well as if the cathode active material capacity exceeds the anode capacity, as a result microscopic deposits appear on the anode surface, which gradually grow.

So, after a short circuit occurs, the battery starts to heat up. When the temperature reaches 70-90 ° C, the ion-conducting protective layer on the anode begins to decompose. And then lithium, built into the anode, reacts with electrolyte, releasing volatile hydrocarbons: ethane, methane, ethylene, etc. But, despite the presence of such an explosive mixture, ignition does not occur, since there is no oxygen in the system yet.
Since the reaction with the electrolyte is exothermic, the temperature and pressure inside the battery continue to rise. When the temperature reaches 180–200 ° C, the cathode material, usually a transition metal oxide with lithium embedded in the crystal, reacts to disproportionation and releases oxygen. This is where the spontaneous combustion takes place and an even sharper temperature jump. In parallel, there is a thermal decomposition of the electrolyte (200-300 ° C), which also produces heat. It looks like this:

And, in the end, graphite enters into reaction with the electrolyte (if it still remains), and when the temperature reaches 660 ° C, the aluminum current collector melts. Above 900 ° C, the temperature usually does not rise, since there is nothing to decompose.
In addition to internal short circuits, there are other causes of spontaneous combustion: overheating of the battery, improper charging / discharging (exceeding the maximum allowable voltage, charging at high currents, too deep discharge), etc. But all these reasons lead to one result: thermal acceleration and decomposition of the electrolyte when interacting with the electrodes. Only the order of the above reactions and their speed differ.
Naturally, battery manufacturers have provided self-ignition protection systems, and the larger and more powerful the battery, the more protection it contains. One type of protection against a small short circuit is a porous separator, which, with a local increase in temperature, becomes impenetrable and prevents, for example, the further growth of dendrites inside the battery. But sometimes the temperature rises too quickly and the separator simply melts, causing the anode to come into contact with the cathode.
The batteries are also equipped with fuses and valves, which, when the pressure and temperature inside increase, either disconnect the electrodes from the circuit, or contribute to the release of accumulated gas. In the latter case, since the gases are flammable, when in contact with oxygen there is a flame outside. An example of the action of safety valves could be observed in an accident involving the Tesla car Model S, where the battery was punctured by a large metal object. Since in Tesla, the battery valves were aimed down on the asphalt and the individual blocks were well isolated from each other, only the front of the battery burned (as Elon Musk said, if the same metal object broke through the gasoline tank, the car would burn completely).

By the way, the thermal insulation of individual units in a large battery is very important. If, in the above example, the Tesla battery did not catch fire completely due to good thermal insulation, in the case of a battery on board the Boeing 787, spontaneous combustion occurred because the blocks were not sufficiently isolated from each other, which led to overheating of the entire system.

Also, lithium-ion batteries are equipped with controllers, sensors, charge balancers, etc. You can read more about battery safety systems here .
As can be seen from this post, the most dangerous component of a battery is an electrolyte, which decomposes into flammable components with increasing temperature. Today scientists are trying to find more stable alternatives: ionic liquids, polymer electrolytes, solid ceramic electrolytes, etc. But this is a separate topic ...
Sources:
»Journal of The Electrochemical Society, 158 3 R1-R25 2011
»Journal of Power Sources 208 (2012) 210– 224
» Www.electrochem.org/dl/interface/sum/sum12/sum12_p037_044.pdf
» Www.powerinfo.ru/accumulator-liion.php
" Www.treehugger.com/cars/elon-musk-letter-explains-why-tesla-model-s-caught-fire.html
Source: https://habr.com/ru/post/372703/
All Articles