A new state of water molecules has been discovered.

Neutronography and computer modeling have shown a unique and unexpected behavior of water molecules under conditions of extreme confinement , phys.org reports .
Researchers at Oak Ridge National Laboratory, in collaboration with Rutherford-Appleton's laboratory, conducted an experiment to find out what happens to water when it is inside minerals. In this way, they wanted to understand what state the fluid takes in rock microcracks, soil, and also during diffusion through cell membranes.
In the course of the experiment, water was placed in hexagonal supersmall channels in the mineral beryl . The diameter of these channels is only 5 angstroms , that is, 10 -10 meters (individual atoms, as a rule, about 1 angstroms in diameter). Subjecting water molecules to the process of tunneling — placing them in these containers — the researchers found that the particles exhibit rather strange behavior, overcoming the potential barrier and starting quantum motion. This state of H 2 O was observed by scientists for the first time and did not correspond to the typical forms of the substance.
')
Previously it was believed that such a phenomenon exists only in quantum mechanics. “In this case, the oxygen and hydrogen atoms are“ delocalized ”and, therefore, they are simultaneously present in the six symmetrical positions of the channel,” said study lead author Alexander Kolesnikov.
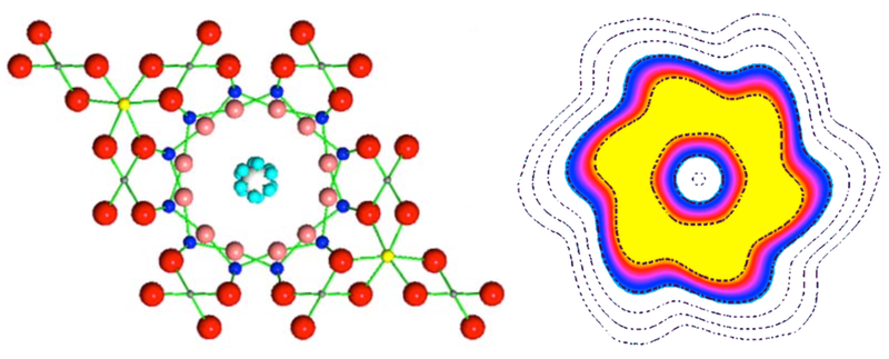
Water rings. One water molecule may be limited inside a hexagonal shaped beryl channel (left). The light blue spheres show the positions of one hydrogen atom in a water molecule, as it happens simultaneously in the six symmetrical positions of the channel. Tunneling between these orientations means that the hydrogen atom is not in one position, but “smeared” in the form of a ring. The right figure on a spatial scale shows the distribution of the electron density of hydrogen, from blue (low) to yellow (highest).
The discovery of this “quantum tunneling” of the state of water molecules in beryl is expected to help scientists understand the thermodynamic properties of water in confined media such as carbon nanotubes, as well as in various geological conditions.
Rutherford-Appleton Laboratory employee Lawrence Anowitz noted that the discovery will cause a discussion in biological, geological and physical and mathematical circles, as scientists will try to explain the mechanism of this phenomenon and understand how it affects their areas.
The researchers also reported that the average kinetic energy of water protons, obtained directly from the neutron experiment, determines their speed at zero degrees Celsius and shows indicators 30% less than in liquid and solid form.
Source: https://habr.com/ru/post/372155/
All Articles