Gene therapy of aging vs pharmacology
The article is not a medical recommendation. Its purpose is to tell about the state of affairs in the industry.
Author: Dmitry Veremeenko . We are also looking for co-authors for the most complete description of the prospects for gene therapy of aging. To be more precise, to create and implement a plan for a radical extension of human life.
All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where the gene is turned off or the path of aging. Compared with other approaches to the extension of life, it is enough to carry out gene therapy only once in a lifetime.
')
Introduction of the telomerase gene (TERT), disruption of the Agtr1a gene, GHRKO knockout, disruption in the genes encoding receptors for IGF-1, over-expression of FGF21, AC5 knock-out, deletion of RIP3, editing PCSK9 gene, over-expression of Klotho, RAGE knockout, over-expressing BubR1 , over-expression of MTH1 - all these are examples of the most effective ways of genetic engineering, allowing animals to prolong life up to 30%.
To achieve more significant results in gene therapy of aging, it is necessary to combine different approaches. Partially repeat the effects of gene therapy can be with the help of farm drugs.
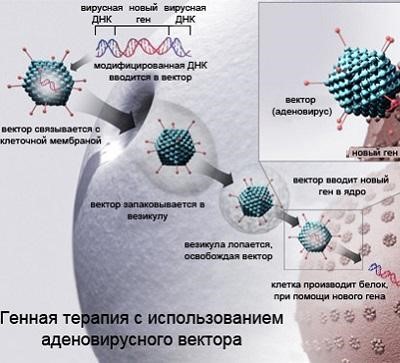
The concept of gene therapy has existed for the past twenty to thirty years. It lies in the fact that the most radical way of dealing with diseases is the destruction of the genetic cause of the disease itself, and not its consequences. Gene therapy is an intervention in the work of the cellular "plant" for the production of proteins. It allows both to activate the work of the desired genes and to “turn off” the harmful ones. In the first case, the gene is delivered into the cell, from which the protein necessary for the therapy of the disease begins to be read. In the second, regulatory RNAs are introduced into the cell, which block the expression of the “harmful” gene. According to the journal Gene Medicine , in 2016, 2,300 clinical trials on gene therapy of various diseases were conducted. These are predominantly cancer (64%), monogenic diseases caused by mutations in a single gene (9.5%), cardiovascular (7.9%) and infectious (7.9%). For a number of diseases, gene therapy has proven to be quite successful.
To date, already 3 gene therapy drugs are allowed for sale. In China, Gendicine, a p53-based drug for the treatment of squamous cell head and neck cancer, was released in 2003, and Oncorine (Oncorine), a virus for the treatment of nasopharyngeal carcinoma, was released in 2006. In Europe, in 2012, the company launched production of the drug Glybera (Glybera), intended for the treatment of hereditary lipoprotein lipase deficiency (LPL) by delivering a gene of the same name.
Severe Combined Immunodeficiency Syndrome (SCID) is a hereditary disorder in children characterized by deeply defective or lack of T cell and B cell functions. SCID often turns out to be fatal during the first year of life, despite carrying out a therapeutic stem cell transplantation or, in the case of adenosine deaminase (ADA) deficiency, an enzyme replacement. In April 2016, the European Agency for Food Safety and the European Medicines Agency approved the gene therapy of this disease with the drug Strimvelis .
On August 30, 2017, the United States Food and Drug Administration (FDA) approved the world's first blood cancer gene therapy . This is Kymriah (tisagenlecleucel) from Novartis Pharmaceuticals, which is based on CAR-T technology and is intended for the treatment of B-cell acute lymphoblastic leukemia in children and young adults under 25 years old, refractory to other methods of treatment or having a relapse of the disease.
The use of the CRISPR / Cas9 genome editing technology opens up new possibilities in gene therapy. CRISPR / Cas9 allows you to very accurately and safely change the DNA of cells. And if you combine the technology of CRISPR / Cas9 with the delivery with the help of adeno-associated viruses, then this seems to allow a systemic effect on the body and it is absolutely safe to change the genome of a very large number of cells.
And in 2016, genetics from Duke University (USA) announced that for the first time in history they were able to successfully carry out gene therapy of an adult mammal (mouse) and cure it of a genetic disease associated with muscle dystrophy. For this, a modified version of the relatively new technology for editing genes CRISPR / Cas9 was used. The technology for editing the CRISPR / Cas9 genes is associated with the use of an adeno-associated virus that helps deliver genetic material to its destination. Using this technology, successful experiments were carried out on editing the genes of individual cells in test tubes and unicellular embryos. Unfortunately, so far the possibility of genetic manipulation on human embryos causes violent disputes.
CRISPR / Cas exceeded all expectations. He allowed with the minimum number of errors both to “turn off” the necessary genes, and to embed new genes in strictly specific regions of the genome.
In December 2015, the scientific group Feng Janga modified this system so that it became completely infallible, which was published in the leading scientific journal Science. Scientists replaced the 3 amino acids “building blocks” of which the protein consists) in the endonuclease Cas9, after which the number of errors of such a system was reduced to almost zero.
The use of CRISP / Cas9 is especially important for gene therapy of aging, where it is necessary to influence the paths of longevity, common to most cells of the body. For gene therapy of aging, up to 2015, no human clinical trials have been carried out, which is not surprising, since aging has not yet been recognized as a disease.
In addition, gene therapy of aging is still very young and developing area. Now all research on gene therapy of aging is carried out on model mice, rats, monkeys and human cell cultures - cells in vitro.
All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where small RNAs are introduced that "turn off" the gene or the pathway of aging. That is, in the first case, something useful is introduced for longevity, and in the second, the harmful is turned off. In the strict sense, only two studies have been conducted on mammalian gene therapy of aging until 2015.
Much more work is modeling gene therapy on transgenic mice. In such studies, the therapeutic gene is not delivered into the body of an adult mouse, and with the help of genetic engineering they create mice whose genome has been changed since birth. Like gene therapy, it allows you to explore how an increase or decrease in the activity of different genes affects the lifespan and aging of the body.
Let's look at what can theoretically be done with the help of gene therapy and genetic engineering to fight aging.
Why do we need gene therapy if you can use anti-aging drugs (geroprotectors)? Compared with other approaches to life extension (for example, geroprotectors or food restriction , prolonging life up to 30-50%), it is enough to carry out gene therapy only once in a lifetime, and you need to take pills all the time and not forget - otherwise the result will not be complete For example, in the work of Andrzej Bartke in 2001, food restriction extended the life of mice by 30% . However, mice consumed a low-calorie diet for up to 670 days in a row - that is, every day, for half of their lives! For most people, this is not very realistic. And in the experiment on gene therapy by Maria Blasco (to be discussed further in this article) in 2012, gene therapy with telomerase led to a slightly smaller effect - mice began to live 20% longer. However, in this work, the mice received only 1 injection of the drug into the blood for a lifetime in a rather old age!
Therefore, if we are talking about the transmission of research on the extension of life per person, gene therapy has an absolute advantage, because it does not reduce the quality of life because of the need for constant treatment - to follow a certain diet every day or to constantly use geroprotectors or other medicines. Also, gene therapy is highly targeted and therefore has the potential for fewer side effects.
In addition, drugs have limitations on bioavailability in various tissues and organs.

The history of telomerase research dates back to 1961. American researcher Leonard Hayflik cultivated human embryo fibroblasts in a test tube and noticed that they can share no more than 50 times, and then grow old. And if you take cells from older donors, then they divide even less.
The scientist suggested that in the cells there is a counter of divisions, limiting their total number. After 10 years, the Russian scientist Alexei Olovnikov offered a hypothetical mechanism for the operation of this counter.
Olovnikov suggested that during cell division, the ends of the chromosomes, called telomeres, are slightly reduced. And when telomeres reach a critical length, the cell stops dividing and ages. In the future, Elizabeth Helene Blackburn, an American cytogenetic scientist, won the Nobel Prize in Physiology or Medicine for 2009 jointly with Carol Grader and Jack Shostak with the phrase “for discovering the chromosome protection mechanisms of telomeres and the telomerase enzyme” according to the theory proposed by Alexey in 1971 Olovnikov.
In non-aging cells (for example, sex and embryonic stem), on the contrary, there must be an enzyme that extends telomeres, allowing the cells to divide almost indefinitely. In addition, it was shown that damage to the telomerase gene greatly shortens the life of model animals and leads to the emergence of premature aging syndrome - progeria.
After the discovery of telomerase, dozens of scientists caught fire in order to make a cure for old age based on it. It would seem that the "inclusion" of telomerase in all cells can make the body immortal. However, concerns soon arose due to the fact that the active synthesis of telomerase was observed in 90% of cancerous tumors. The question arose: will not the activation of telomerase lead to the risk of malignant transformation?
In addition, it turned out that cell aging is not always accompanied by a reduction in telomeres. For example, in the case of epithelial cells of the oral mucosa or cornea of the human eye. This suggested that telomerase activation alone may not be enough to rejuvenate the entire body. Before proceeding to gene therapy, the effects of telomerase were investigated in transgenic mice. It turned out that if you "turn on" the TERT gene in all cells of the mouse, then the lifespan is increased by 40%! However, the constant activity of telomerase increased the risk of cancer. Therefore, there was a question about how to activate telomerase work for a shorter period.
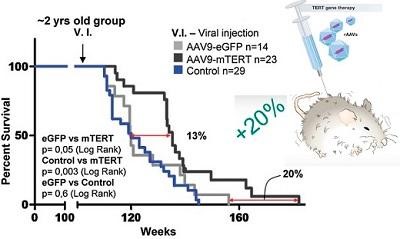
This was done in the work of Maria Blasco 2012 (see chart). The telomerase gene was delivered to the mouse using an adeno-associated virus (AAV9) capable of providing systemic delivery. Adeno-associated viruses are characterized by high safety: they do not embed the delivered gene into the host genome and therefore do not lead to mutagenesis (there is no risk of cancer). In addition, they almost do not cause an immune response. At the same time, TERT genome therapy was completely safe: the risk of cancer in mice did not increase. Two-year-old mice were given one injection, with adenovirus, to which the telomerase gene was inserted. This prolonged the life of the mice by 20% (as shown in the graph above). And this theoretically can allow people aged 40-50 years to give one injection of such a medicine and prolong their life by another 8-12 years.
Today, telomerase can be stimulated with drugs. An interesting study in this area was conducted by scientists from the University of Ljubljana (Slovenia) in 2016 after a series of successful clinical trials on vessel rejuvenation with low doses of valsartan and fluvastatin. This time they measured the activity of telomerase after vascular rejuvenation in the blood samples of 130 patient patients.
So a one-month course of valsartan 20 mg + fluvastatin 10-20 mg significantly increases the activity of telomerase by 3.28 times , which significantly correlates with the improvement of endothelial function (vascular rejuvenation) and reduction of inflammation in the blood vessels. And this increased level of telomerase persists, gradually decreasing, for another six months. But how effective such an increase in telomerase affects telomeres remains to be determined.
 It is important to know that telomeres may not necessarily prolong our life if such therapy is not done at the right time and for too long.
It is important to know that telomeres may not necessarily prolong our life if such therapy is not done at the right time and for too long.
Moreover, telomerase stimulation alone can not lengthen telomeres. Telomerase activity hardly changes with age - look at the graph on the left. And the telomeres are still shrinking.
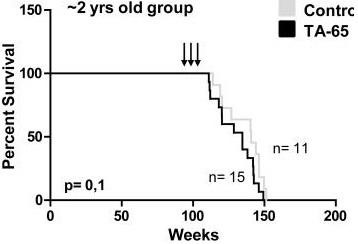 Also today there is a drug on the market that increases telomerase activity - TA-65. He is very expensive, and the life of the mice did not prolong the research. Look at the graph on the left. In a study of 2011, scientists from the Spanish National Cancer Center began to give TA-65 long-lived two-year-old mice to increase telomerase, as in the previous study. Only in a previous study, mice were injected for gene therapy. But TA-65 did not prolong the life of mice, unlike gene therapy (see chart on the left), and turned out to be absolutely useless for prolonging life and slowing aging.
Also today there is a drug on the market that increases telomerase activity - TA-65. He is very expensive, and the life of the mice did not prolong the research. Look at the graph on the left. In a study of 2011, scientists from the Spanish National Cancer Center began to give TA-65 long-lived two-year-old mice to increase telomerase, as in the previous study. Only in a previous study, mice were injected for gene therapy. But TA-65 did not prolong the life of mice, unlike gene therapy (see chart on the left), and turned out to be absolutely useless for prolonging life and slowing aging.
In 2011, scientists from the University of Texas studied telomeres and telomerase in cell cultures of more than 60 species of mammals . The role of telomeres in longevity was not so obvious ... Studies show (when comparing about 60 species of mammals), the longer the telomeres of a species are, the faster DNA mutations accumulate in it, the more cancers and shorter the lifespan. Telomere length is inversely correlated with longevity. This suggests that the result of telomerase life extension, which was obtained in mice with a single injection, may not prolong the life of people. The question on telomeres for people remains open.
Conclusion: In the future, theoretically, we can, through the introduction of the telomerase gene (TERT) at the age of 40-50 years with a single injection, increase the length of telomeres, but this therapy alone is not enough. Most likely, we must find a combination of gene-therapeutic effects in order to significantly prolong human life. Today we can imitate the effect with one-month therapy 1 time in half a year with a combination of drugs of valsartan 20 mg + fluvastatin 10-20 mg , or telmisartan + atorvastatin 10 mg. At least, these drugs in combination are able to stimulate telomerase itself.
Angiotensin II receptor antagonists, or AT1 receptor blockers, are one of the newer groups of antihypertensive drugs (drugs for treating blood pressure). Such drugs include all drugs of the Sartan group (for example, Telmisartan) .
As has been shown in studies , these drugs greatly prolong the life of animals and even humans. For example, according to a 2007 study conducted by scientists from the University of Buenos Aires (Argentina), one of the Sartans losartan, when given to 40 Wistar rats, he extended their maximum life expectancy by 18% compared to the other 40 rats in the control group .
 AT1 receptors can also be affected genetically by acting on the Agtr1a gene. This solves the problem of drug delivery to all tissues and organs. A unique study was conducted by researchers from the Institute of Pharmacological Research (Italy) and Duke University (USA) in 2009. As we can see on the graph, disruption of the Agtr1a gene encoding angiotensin receptor AT1a prolongs the life of 20 transgenic mice compared to 10 wild-type mice by 26%. Judging by research, functional aging of organs and tissues slowed down.
AT1 receptors can also be affected genetically by acting on the Agtr1a gene. This solves the problem of drug delivery to all tissues and organs. A unique study was conducted by researchers from the Institute of Pharmacological Research (Italy) and Duke University (USA) in 2009. As we can see on the graph, disruption of the Agtr1a gene encoding angiotensin receptor AT1a prolongs the life of 20 transgenic mice compared to 10 wild-type mice by 26%. Judging by research, functional aging of organs and tissues slowed down.
This gene therapy can be used in the elderly to prolong life. The effect on AT1 receptors for people with high blood pressure is especially relevant.
Conclusion: In the future, using gene therapy, we can disrupt the gene encoding angiotensin receptor AT1 and prolong human life for 5-15 years (such dates are taken by extrapolating the results obtained in animals) depending on the patient's condition. Today, a partial effect (due to incomplete bioavailability in all tissues of the body) can be achieved by therapy of course treatments in low doses of the drug telmisartan at a dose of 10 mg per day , if there is no high blood pressure - otherwise the dose is higher.
 In humans, growth hormone regulates the level of IGF-1 (insulin-like growth factor 1 type). It happens like this. HGH is secreted in the anterior lobe of the pituitary gland. The growth hormone then binds to receptors in the liver. Due to binding to growth hormone receptors, insulin-like growth factor 1 type (IGF-1) begins to be secreted in the liver.
In humans, growth hormone regulates the level of IGF-1 (insulin-like growth factor 1 type). It happens like this. HGH is secreted in the anterior lobe of the pituitary gland. The growth hormone then binds to receptors in the liver. Due to binding to growth hormone receptors, insulin-like growth factor 1 type (IGF-1) begins to be secreted in the liver.
If growth hormone receptors are not blocked or “broken,” the higher the growth hormone, the higher the IGF-1. Today, one of the most proven in almost all animal species ( www.karger.com/Article/Abstract/212538 ; www.ncbi.nlm.nih.gov/pubmed/19590001 ) the life extension method is the reduction of IGF-1 (insulin is a similar factor type 1 growth) with the help of fasting , or with the help of the FMD diet, developed by the scientist Walter Longo from the University of Southern California (USA) in 2015.
But for this you need to follow a diet all your life, which not everyone can do.
IGF-1 is a hormone that binds to IGF-1 receptors. And so, if the IGF-1 receptors are genetically disturbed, then the IGF-1 deficiency can be partially imitated.
According to a 2008 study made by scientists from the Albert Einstein Institute (USA), a violation in the genes encoding receptors for IGF-1 in 197 Jewish descendants of Ashkenazia's long-livers (105 women and 92 men) probably explains their longevity phenomenon. It seems that superdolongers, who live for more than 100 years, had an optimal (not as high as other people) level of IGF-1 in childhood and adulthood, or IGF-1 had a high level, but at the same time a genetically determined defect of receptors to IGF-1.
What if IGF-1 optimization and mortality reduction is just a coincidence. After all, the longevity is not so much.We would be told about such a relationship by research on large groups of people. There are such studies.
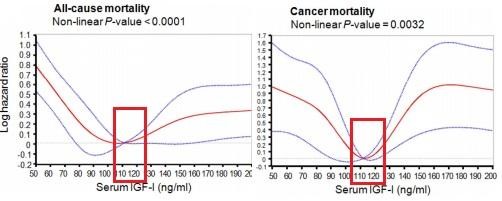
Research data suggests that there is an IGF-1 level that is higher and lower than the risk of death from cancer and other causes in humans.
This range is from about 105 ng / ml to 120 ng / ml. (see the chart on the left). This fact was shown in their studies by Swedish scientists from the University of Gothenburg in 2012 as a result of a study of 2901 patients, as well as by American scientists from the Gerontology School of Davis at the University of Southern California in 2014. In people aged 50 to 65 years, IGF-1 at a level of 105 to 120 ng / ml was associated with the lowest total mortality in the next 18 years.
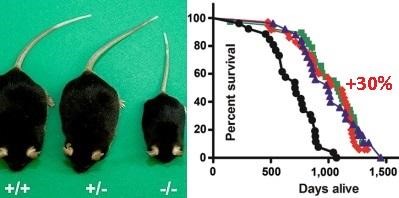 Knockout in mice of the gene encoding GHRKO growth hormone receptors (selective removal of growth hormone receptors) prolongs the life of transgenic miceby about 30% compared with wild-type mice.
Knockout in mice of the gene encoding GHRKO growth hormone receptors (selective removal of growth hormone receptors) prolongs the life of transgenic miceby about 30% compared with wild-type mice.
On the chart: the black color of the line is a wild type mouse. The blue chart is wild-type mice that were undernourished to reduce their IGF-1 (they lived 30% longer than wild-type mice).
The red line is a mouse with a genetically low IGF-1, since they have knocked out the gene encoding the GHRKO growth hormone receptors. They lived 30% longer than wild-type mice).
The green line is the same mouse with a knockout of the gene encoding the GHRKO growth hormone receptors, but which are also under-nourished - their under-feeding did not give any advantages , -1 . ? , -1 , , .
 , , .
, , .
GHR-KO 11C, (Andrzej Bartke) — , ().
6 , 1819 . - , -1.
25 2003 Longevity Prize — . , -1 500 .
60 , -1 30 3-4 (500 3 ).
: , GHRKO ( ), , -1 5-17. , . , -1 2- 500 .
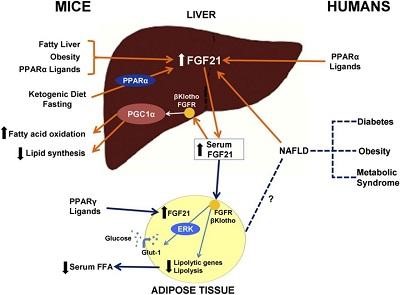 Fibroblast growth factor 21 (FGF21) is a hormone secreted by the liver during fasting periods in order to help the body adapt to nutritional deficiencies.
Fibroblast growth factor 21 (FGF21) is a hormone secreted by the liver during fasting periods in order to help the body adapt to nutritional deficiencies.
FGF21 is also synthesized by the liver in response to diabetes and many other health problems, and even with alcohol. FGF21 blocks growth hormone receptors due to which the synthesis of IGF-1 by the liver is reduced.
Lower than usual IGF-1 in many studies has greatly prolonged the life of animals and reduced mortality in humans. It would be interesting to check whether it is possible to simply genetically force the body to secrete more FGF21. Will it prolong life significantly? As it turned out - you can.
By the way, Metformin is able to simulate the effect of increasing FGF21 at a dosage of 500 mg per day.
2013 () , FGF21 .
 () 2012 , FGF21 ( 21) ( ) ( ), .
() 2012 , FGF21 ( 21) ( ) ( ), .
: FGF21 ( 21) . FGF21 Metformin at a dosage of 500 mg per day. But it is fatally dangerous to use metformin to people with elevated above normal creatinine in blood tests. Therefore, before use, the doctor is obliged to prescribe a blood test for creatinine. Otherwise, metformin is considered a drug with a high safety profile.
, . , , , .
— — . . AC5 ( www.ncbi.nlm.nih.gov/pubmed/18205980 ; www.ncbi.nlm.nih.gov/pubmed/19922557 ). (, ).
It should be noted that it is not possible to combine the genetic blockade of growth hormone receptors and Adenylate cyclase type 5 (AC5). As shown in a 2013 study (University of Medicine and Dentistry of New Jersey - USA), mice in such an experiment died within a month, as if they were starving. These mice were blocked with AC5 and reduced calorie intake. Therefore, either starve or completely block the AC5. Either starve and reduce the excessive activity of AC5, but not block it completely.
— , - («flight or fight»); , , , , , . , , , .
Stephen F. Vatner «» 5 (AC5) — , — β-.
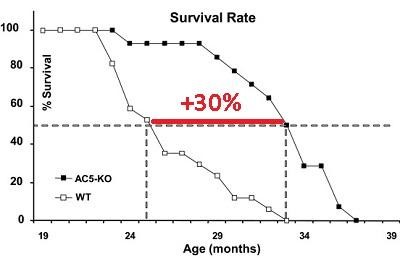 Such receptors are present on almost all cells of the body. Look at the chart. The knockout of AC5 greatly extended the median lifespan of transgenic mice by 30% compared with wild-type mice, protected the heart from aging, protected it from type 2 diabetes and obesity.
Such receptors are present on almost all cells of the body. Look at the chart. The knockout of AC5 greatly extended the median lifespan of transgenic mice by 30% compared with wild-type mice, protected the heart from aging, protected it from type 2 diabetes and obesity.
At the molecular level, these effects were due to the fact that the inactivation of AC5 launched the path of resistance to stress - Raf / MEK / ERK.
As a result, the cell produced a whole set of protective molecules, triggering the mechanisms of cell survival. In addition, it is known from earlier works that the action of adrenaline on a cell through β2 -adrenoreceptors directly causes DNA damage.
Thus, it can be assumed that "turning off" the AC5 gene of mice also contributed to increased genome stability.
, AC5 , . FDA -9-β-- (Vidarabine AraAde), 5 .
, 5 — , .
, , 5 .
, — . 1988 .
Kaplan, using the example of primates, showed that if a group of primate males was assembled, then for some days the monkeys would have a social hierarchy. The worst place in such a hierarchy is below. Primate males in a subordinate position show a number of indicators of chronic stress. Often they develop atherosclerosis. When scientists gave primate males that are at the bottom of the social hierarchy (risk group), beta-blocker propranolol , which suppresses the activity of the sympathetic nervous system, atherosclerosis of the vessels did not develop.
It turned out that the sympathetic nervous system negatively affects the development of atherosclerosis and is involved in problems with the heart and blood vessels due to stress. Emotional stress realizes itself through the sympathetic (adrenergic) autonomic nervous system, which connects the control centers of our brain and internal organs. Including - with the immune, bone marrow and others. Atherosclerosis is the main factor that leads to the greatest number of deaths in developed countries from heart attack and heart stroke.
A randomized, double-blind, placebo-controlled study in 1983 , organized by Goldstein S and colleagues, showed that propranolol therapyin 3837 patients with acute myocardial infarction reduces mortality from cardiovascular diseases (cause of death # 1 in the world).
A three-year observation of 60-69 years old patients from the United States who suffered a myocardial infarction, published in 1997 , showed that propranolol reduced overall mortality in such patients by 35-37%. In addition, propranolol caused a decrease in signs of disease and aging of the heart (a significantly greater increase in the left ventricular ejection fraction and a significantly greater decrease in the mass of the left ventricular myocardium).
Conclusion: , 5- . , , , , -. , , . , , , !!! 5 ( 50%) in mini-doses, significantly lower than high doses used in the treatment of cognitive disorders.
, - , . , , ( ) , , .
«−/−» — . — . ( ) . , , . , ,
[www.ncbi.nlm.nih.gov/pmc/articles/PMC4066890].
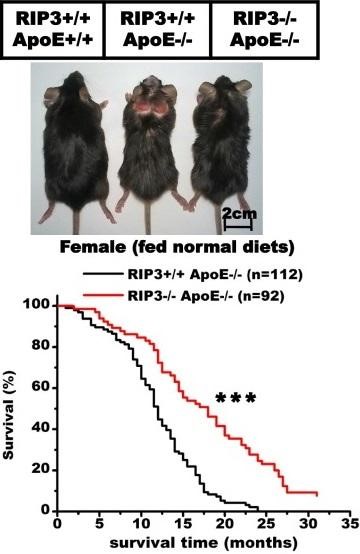 Apoe . 2015 , , . , , ApoE +/+ — ApoE, . . ApoE -/-, . ApoE -/- RIP3 (RIP3-/-), , , ApoE +/+ .
Apoe . 2015 , , . , , ApoE +/+ — ApoE, . . ApoE -/-, . ApoE -/- RIP3 (RIP3-/-), , , ApoE +/+ .
, ApoE -/- - RIP3 +/+ 25 . RIP3 (RIP3 -/-), 32 — .
RIP3 , . . — , .
RIP3 , , .
50% ApoE -/- RIP3 +/+ , ApoE -/- RIP3 -/- 88% .
RIP3 NF-κb , ( ). : RIP3 +/+ . RIP3 () .
From the previous data, the reader can conclude that after RIP3 knockout, although atherosclerosis will not develop, there will be a danger of cytomegalovirus. After all, RIP3 causes apoptosis (self-destruction) to protect against viral infection. Human cytomegalovirus (CMV) is a major virus that causes birth defects and serious problems in immunocompromised persons. CMV is also associated with atherosclerosis (vascular disease). Previous studies have shown that the induction of mTOR independent autophagy can block the replication of various DNA and RNA viruses.
In 2015, scientists from the University of California (USA) on cell lines proved that mTOR activation of independent autophagy with trehalose could be another method for treating CMV. Same resultswere obtained in 2008 at the University of Paris (France). As an mTOR inducer of independent autophagy, trehalose also showed itself in clinical trials. Now there is the relevance of conducting clinical trials of intravenous trehalose for the treatment of CMV. Trehalose is a sugar that can enter our regular diet instead of sugar, as it is a potential geroprotector (a means of prolonging life).
 By tradition, let's see if it is possible to crush RIP3 with the means available to us today. It turns out you can. So, according to a 2016 study by scientists from Peking University, low doses of aspirin (L-ASA), though not reducing necrosis through RIP3 to the level of healthy mice (control), reduce necrosis at least partially compared to SAP. Look at the chart.
By tradition, let's see if it is possible to crush RIP3 with the means available to us today. It turns out you can. So, according to a 2016 study by scientists from Peking University, low doses of aspirin (L-ASA), though not reducing necrosis through RIP3 to the level of healthy mice (control), reduce necrosis at least partially compared to SAP. Look at the chart.
0,2. — 0,8. — 0,3 ( , ).
, , ? 2002 , , , , .
RIP3 (necrosulfonamide).
: RIP3 . , , RIP3 : 250 1 .
Cardiovascular diseases are the number one killer worldwide. In June 2014, scientists at Harvard University were able to edit the genes in> 50% of the liver cells of an adult mouse - using adenovirus with Cas9 / CRISPR.
It was possible to make changes in the PCSK9 gene using a single intervention. People with such changes in this gene have lower levels of bad cholesterol and are less likely to suffer from cardiovascular diseases. Thus, it was shown that only one injection can reduce the level of bad cholesterol and in the future do without drugs to reduce the level of cholesterol - statins .
Twenty years ago, in 1997, Japanese and American scientists, led by Yo-ichi Nabeshima, discovered a new longevity gene and named it in honor of the Greek goddess of fate, the weaving thread of life - Klotho. His "off" in transgenic mice caused accelerated aging syndrome.
After 7 years, the same researchers found that the activation of klotho significantly prolongs the life of mice - males by 20-30%, and females - up to 19%.
Klotho is produced in the kidneys and blood vessels of the brain and is carried by the blood through the body like a hormone. It systemically affects most cells in the body - binds to receptors on their surface and inhibits insulin and IGF-1 signaling (one of the main ways of aging).
Three years after the discovery of the klotho gene, in 2000, the University of Gumma in Japan carried out the first work on gene therapy , but not from aging, but from chronic vascular disease, atherosclerosis. Ryozo Nagai and colleagues systematically introduced this gene to rats and achieved improved vascular status and lower blood pressure.
In 2014, a joint study of Japanese and American scientists from the Jichi Medical University, as well as the University of California and Boston showed that certain variants in the klotho gene (SNP Rs9536314) are associated with longevity and high mental abilities.
 To understand how Klotho influences us, consider a study conducted in 2005 by scientists from the University of Texas (USA), which showed that mutagenic mice with a Klotho gene with a defect looked normal for about 3-4 weeks of age and then began to age fast.
To understand how Klotho influences us, consider a study conducted in 2005 by scientists from the University of Texas (USA), which showed that mutagenic mice with a Klotho gene with a defect looked normal for about 3-4 weeks of age and then began to age fast.
For a month, they showed such bright signs of aging: skin atrophy, osteoporosis, atherosclerosis, and emphysema. About two months later, the mice died.
 In other studies, scientists from the University of Texas (USA) have bred a second type of mutagenic mouse , in which the Klotho gene produces significantly more protein than normal mice. These mice lived on 19% - 30% longer than their usual relatives (see the graph on the left).
In other studies, scientists from the University of Texas (USA) have bred a second type of mutagenic mouse , in which the Klotho gene produces significantly more protein than normal mice. These mice lived on 19% - 30% longer than their usual relatives (see the graph on the left).
Scientists note that Klotho is the rarest case in mammalian biology, where a single gene has such a significant effect on life expectancy. Overexpression of the klotho protein causes insulin resistance to IGF-1, imitating starvation.
Scientists from the University of California (USA) in 2015 showed that the level of klotho protein naturally decreases as the body ages, which leads to a deterioration in cognitive abilities. Increased expression of the klotho protein is associated with better cognitive functions in normal healthy people.
While we are not able to increase the level of klotho in the body with the help of genetic engineering, we can do this with the help of drugs. A randomized controlled study on people aged about 60 years , published in 2015 by the Royal College of London (United Kingdom) in the journal CJASN, showed that treatment with valsartan or losartan (drugs for high blood pressure) increases klotho longevity protein expression.
And in 2010, a study of the Nagoya University (Japan) was published in the American Journal of Nephrology , where it was shown how activated carbon increases the expression of klotho longevity protein in rats. Activated carbon inhibits kidney overload with indoxyl sulfate, which leads to an increase in kloto. Indoxyl sulfate is the product of tryptophan metabolism in the intestine using microflora to indole and further metabolism in the liver to indole sulfate. But activated carbon can adsorb indole sulphate and intestinal bacteria that are involved in the metabolism of other kidney toxins. Indole has a toxic effect on the cells of the kidneys and blood vessels - increasing oxidative stress in the kidneys and in the vessels. As the glomerular filtration rate of the kidneys decreases with aging, the renal clearance of indoxyl sulfate decreases in humans and in rats, which leads to its increased content, toxicity and, consequently, to a decrease in klotho. Indoxyl sulfate is a uremic toxin that accelerates the progression of chronic renal failure.
Conclusion: In the future, we may be able to increase Klotho expression and prolong life with the help of genetic engineering, depending on the time of the intervention and gender. Today, some blood pressure medications ( valsartan and losartan ) have been shown to increase klotho in humans, and also increase the use of activated charcoal , at least in rodents.
The accumulation of glycation end products (AGE) is one of the causes of human aging, as well as many age-related diseases: atherosclerosis, myocardial infarction, congestive heart failure, diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, Alzheimer's disease, psoriasis. RAGE is a receptor that binds to the final glycation products (AGE). The binding of AGE and this receptor (RAGE) leads to the formation of reactive oxygen species (ROS) with the subsequent activation of the oxidatively sensitive NF-κB transcription factor in the vascular wall and not only regulating the expression of inflammatory and “responding to damage” genes, and directly to the RAGE gene . The negative effect of AGE products is realized not only in the walls of the vessels, but also in neurons and even in bone tissue.
In 2014, thanks to scientists from George Mason University, it became clear that birds do not have RAGEs , and it is possible that they have a longer lifespan at higher body temperature and blood glucose levels than mammals of the same mass.
Birds do not suffer from diabetes in normal conditions. In addition, the plasma of birds contains a lower concentration of methylglyoxal. Methylglyoxal is the precursor to the end products of glycation.
 A 2007 study on mice (Icana School of Medicine, Mount Sinai Medical Center, USA) showed that a diet with a reduction in mice of one of the most common end products of glycation (carboxymethyl-lysine) from about 30 weeks old (from youth humans) was sufficient to significantly increase the average and maximum life expectancy of animals (by 15% and 6%, respectively).
A 2007 study on mice (Icana School of Medicine, Mount Sinai Medical Center, USA) showed that a diet with a reduction in mice of one of the most common end products of glycation (carboxymethyl-lysine) from about 30 weeks old (from youth humans) was sufficient to significantly increase the average and maximum life expectancy of animals (by 15% and 6%, respectively).
The graph shows that mice with a reduction in the diet of the end products of glycation lived much longer. In addition, in mice with low levels of glycation end products in the diet, body weight was significantly reduced. This shows that excess weight is not only a consequence of calories in food, but also a consequence of a high consumption of glycation end products.
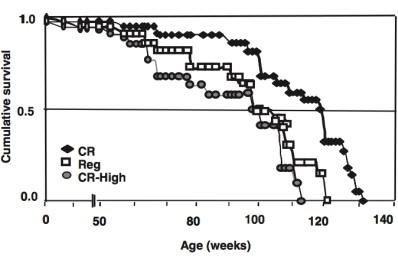 However, as shown in the graph on the left (from a 2008 study by scientists from the University of Miami - USA), if in low-calorie diets in mice provide the same amount of glycation end products as in normal diets, then life extension is not observed.
However, as shown in the graph on the left (from a 2008 study by scientists from the University of Miami - USA), if in low-calorie diets in mice provide the same amount of glycation end products as in normal diets, then life extension is not observed.
But you can not reduce the end products of glycation, but simply knock out with the help of genetic engineering RAGE receptors. Genetic blockade of RAGE receptors in mice has been carried out more than once by researchers from Brigham Young University (USA) in 2016, as well as in the same year by Swedish scientists from Uppsala University . Such blockades led to positive health outcomes for mice. How this will affect the prolongation of the life of the mice apparently has not been verified.
Translated into our diet - it will look like this. If you reduce the calories, but at the same time eat fried, baked food, as well as a lot of sweets and flour, then there will be no health benefits.
At the same time, even if you do not reduce calories in the diet, but completely exclude fried, baked meat from the diet, eat meat only in boiled form, and vegetables only in cheese, do not eat sweets and foods with a high glycemic index, add means inhibitors of final products glycation ( metformin 500 mg per day, vitamin B6 5-20 mg per day, raw broccoli cabbage 100 grams per day), it is possible to increase the lifespan. According to Japanese scientists from Kurume University in 2008, telmisartan, also a drug, reduces the ability of RAGE to bind to the end-products of glycation in hypertensive patients.
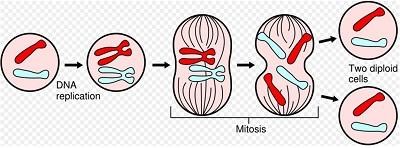 The researchers found that a protein called BubR1 reduces the risk of cancer and by 15% and the maximum lifespan of experimental mice.
The researchers found that a protein called BubR1 reduces the risk of cancer and by 15% and the maximum lifespan of experimental mice.
BubR1 protein helps cells form chromosomes in the process of division. When BubR1 expression is reduced, chromosomes can not properly divide during mitosis, and cells with defective chromosomes are formed.
BubR1 protein is studied superficially, but it is known that most cancer cells are formed precisely because of defective chromosomes, or rather, their different numbers. This phenomenon is called aneuploidy (a change in karyotype, in which the number of chromosomes in cells is not a multiple of the haploid set), and it is common to all cancer cells, although it is not known for certain whether aneuploidy is the main cause of cancer.
 However, aneuploid cells have too few or too many chromosomes, and BubR1 protein plays a key role in this phenomenon.
However, aneuploid cells have too few or too many chromosomes, and BubR1 protein plays a key role in this phenomenon.
In the course of the experiments, in 2013, American scientists from the Mayo Clinic in Rochester, Minnesota, bred using transgenic mice (T23) with the increased expression of BubR1 by genetic engineering .
Then the experimental mice were subjected to the action of irreversibly causing tumors of the lungs and skin.
As a result, it turned out that oncological diseases “earned” only 33% of transgenic mice (T23) with over-expression of BubR1.
In this case, the disease in mice with hyper-BubR1 occurred later than in wild-type mice - after about 2 years, and the death rate from cancer was several times lower - 15% versus 40% (see the first graph on the left). Moreover, the life expectancy of rodents increased by 15% (see the second graph on the left), and their physical form impressed even scientists. So, the mice ran through the treadmill 200 m, and not 100, like normal mice.
Perhaps Van Dersen and his colleagues took the first step toward creating new cancer treatments and ways to delay aging. Interestingly, in 2005, scientists from the University of Pennsylvania (USA) showed that the antibiotic doxycycline , which is seen as a powerful drug for the treatment of certain types of cancer, can increase the expression of BubR1.
Conclusion: In the future we will be able to increase the expression of BubR1 with the help of genetic engineering and prolong the life of years by 10, as well as delay the development of many types of cancer. Today in clinical trials, the antibiotic doxycycline , which is a potent inhibitor of cancer cell proliferation in animals, is being tested for the treatment of certain types of cancer. And doxycycline, is just the activator of BubR1.
A characteristic feature and, apparently, one of the main causes of aging is the accumulation of errors and DNA mutations. In addition, numerous diseases of premature aging - progeria - also arise as a result of excessive accumulation of genomic damage.
During the life of the DNA is constantly damaged by various influences. From the external environment it is affected by temperature, UV radiation, chemical mutagens and viruses. But the internal processes of the cell itself increase the genomic instability: DNA is damaged by active forms of oxygen from mitochondria, and replication systems (DNA doubling) and DNA repair often work inaccurately and introduce errors themselves.
As a result, with age, a whole spectrum of disorders accumulates in the DNA: breaks, point mutations, transfer of pieces of DNA from one place to another, sticking of chromosomes, inserting viruses and transposons into the genome. If there is too much damage, the cell is forced to go in one of 3 ways: start the processes of cell suicide (apoptosis), become senescent or turn into cancer. All these processes (if they are subject to many cells) are extremely harmful to the body.
The most studied way to increase the stability of the genome is the activation of DNA repair systems. This approach is used in most of the work on gene therapy.
 In 2013, Italian scientists led by Gemma Calamandre investigated transgenic mice with over-expression of the MTH1 gene . Protein MTH1 is involved in the repair of DNA damage and RNA caused by reactive oxygen species.
In 2013, Italian scientists led by Gemma Calamandre investigated transgenic mice with over-expression of the MTH1 gene . Protein MTH1 is involved in the repair of DNA damage and RNA caused by reactive oxygen species.
It corrects defective nucleotides - guanidines oxidized at the eighth carbon atom (of the four letters on which this DNA is written is denoted by “G”). It turned out that increased expression of MTH1 prolongs the life of transgenic mice by up to 22% (see graph).
In addition, in these mice, genomic damage accumulated with age much less. Their behavior also changed: the mice became less disturbing and more curious.
Genetic engineering is not available in humans today. Nevertheless, according to 2014 data from Japanese scientists at Tottori University, we can slightly affect repair and protect DNA and increase MTH1 with the help of magnesium. One of the best ways to maintain the necessary level of magnesium in the blood is supplementation of magnesium citrate 300-400 mg of elemental magnesium per day.
French scientist Elena Martin and her colleagues in 2008 demonstrated in their study that magnesium is important for the functioning of many enzymes involved in copying and restoring DNA damage. Chronic magnesium deficiency in food leads to oxidative stress and accumulation of DNA damage, which was also shown by scientists from Duke University (USA) in 1988, scientists from the University of Karlsruhe (Germany) in 2001.
When examining patients from the University Hospital San Juan de Reus (Spain) in 2014, it was revealed that people with the highest levels of magnesium in the plasma within the normal range and the highest consumption of it, died 34% less often - that is, they had 34% lower mortality from all causes.
Conclusion: In the future, with the help of genetic engineering or gene therapy, we will be able to increase the expression of MTH1 and prolong life for 6-12 years, protecting DNA from damage. And today, to partially achieve the same effect, we can use magnesium citrate 300-400 mg as supplements. Moreover, magnesium showed a decrease in mortality in humans by 34%.
There is a lot of evidence that the impact on several paths of longevity at once gives a greater effect on life expectancy than the impact on them separately.
An extraordinary survivor - a naked digger - a rodent that lives 10 times longer than a mouse, differs from it by activating a whole set of longevity pathways: this is increased resistance to stress, protection against cancer, stability of protein molecules, protection against neurodegeneration.
Thus, in order to develop a gene therapy that drastically prolongs life, it is necessary to act simultaneously on several molecular paths.
It is necessary to combine those gene therapy approaches that are being developed now to increase the stability of the genome, rejuvenate the hypothalamus, destroy senescent cells, increase resistance to stress, improve mitochondrial function, and maintain stem cell niches. It is important to remember that not individually tested combination of effects in studies useful mechanisms can shorten life.
If now single-gene therapy does not lead to an increase in life expectancy of more than 20-30%, then we hope that in the future, due to the complex therapy of aging, it will be possible to achieve truly fantastic results.
Laboratories in the USA , laboratories in China and the UK have announced plans or ongoing research on the use of the crispr genome editing technology for human embryos. True, so far these attempts have failed. But it all starts with trial and error.
On August 30, 2017, the United States Food and Drug Administration (FDA) approved the world's first blood cancer gene therapy .
In March 2017, French scientists reported on successful clinical studies of gene therapy for the treatment of sickle cell anemia.
The Committee of the American National Academy of Sciences and the National Academy of Medicine gave support for editing the human embryo genome as early as 2017 . But only for serious diseases and under strict control.
findings
1. All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where the gene is turned off or the path of aging.
2. Compared with other approaches to the extension of life, it is enough to carry out gene therapy only once in a lifetime.
3. Introduction of the telomerase gene (TERT), disruption of the Agtr1a gene, GHRKO knockout, disruption in the genes encoding IGF-1 receptors, over FGF21 expression, AC5 knockout, RIP3 deletion, PCSK9 gene editing, over Klotho expression, over RAGE knockout, over BubR1, MTH1 — , .
4. , .
5. : , , , 6, , , + , , FMD, , , , , .
Author: Dmitry Veremeenko . We are also looking for co-authors for the most complete description of the prospects for gene therapy of aging. To be more precise, to create and implement a plan for a radical extension of human life.
All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where the gene is turned off or the path of aging. Compared with other approaches to the extension of life, it is enough to carry out gene therapy only once in a lifetime.
')
Introduction of the telomerase gene (TERT), disruption of the Agtr1a gene, GHRKO knockout, disruption in the genes encoding receptors for IGF-1, over-expression of FGF21, AC5 knock-out, deletion of RIP3, editing PCSK9 gene, over-expression of Klotho, RAGE knockout, over-expressing BubR1 , over-expression of MTH1 - all these are examples of the most effective ways of genetic engineering, allowing animals to prolong life up to 30%.
To achieve more significant results in gene therapy of aging, it is necessary to combine different approaches. Partially repeat the effects of gene therapy can be with the help of farm drugs.

The concept of gene therapy has existed for the past twenty to thirty years. It lies in the fact that the most radical way of dealing with diseases is the destruction of the genetic cause of the disease itself, and not its consequences. Gene therapy is an intervention in the work of the cellular "plant" for the production of proteins. It allows both to activate the work of the desired genes and to “turn off” the harmful ones. In the first case, the gene is delivered into the cell, from which the protein necessary for the therapy of the disease begins to be read. In the second, regulatory RNAs are introduced into the cell, which block the expression of the “harmful” gene. According to the journal Gene Medicine , in 2016, 2,300 clinical trials on gene therapy of various diseases were conducted. These are predominantly cancer (64%), monogenic diseases caused by mutations in a single gene (9.5%), cardiovascular (7.9%) and infectious (7.9%). For a number of diseases, gene therapy has proven to be quite successful.
To date, already 3 gene therapy drugs are allowed for sale. In China, Gendicine, a p53-based drug for the treatment of squamous cell head and neck cancer, was released in 2003, and Oncorine (Oncorine), a virus for the treatment of nasopharyngeal carcinoma, was released in 2006. In Europe, in 2012, the company launched production of the drug Glybera (Glybera), intended for the treatment of hereditary lipoprotein lipase deficiency (LPL) by delivering a gene of the same name.
Severe Combined Immunodeficiency Syndrome (SCID) is a hereditary disorder in children characterized by deeply defective or lack of T cell and B cell functions. SCID often turns out to be fatal during the first year of life, despite carrying out a therapeutic stem cell transplantation or, in the case of adenosine deaminase (ADA) deficiency, an enzyme replacement. In April 2016, the European Agency for Food Safety and the European Medicines Agency approved the gene therapy of this disease with the drug Strimvelis .
On August 30, 2017, the United States Food and Drug Administration (FDA) approved the world's first blood cancer gene therapy . This is Kymriah (tisagenlecleucel) from Novartis Pharmaceuticals, which is based on CAR-T technology and is intended for the treatment of B-cell acute lymphoblastic leukemia in children and young adults under 25 years old, refractory to other methods of treatment or having a relapse of the disease.
The use of the CRISPR / Cas9 genome editing technology opens up new possibilities in gene therapy. CRISPR / Cas9 allows you to very accurately and safely change the DNA of cells. And if you combine the technology of CRISPR / Cas9 with the delivery with the help of adeno-associated viruses, then this seems to allow a systemic effect on the body and it is absolutely safe to change the genome of a very large number of cells.
And in 2016, genetics from Duke University (USA) announced that for the first time in history they were able to successfully carry out gene therapy of an adult mammal (mouse) and cure it of a genetic disease associated with muscle dystrophy. For this, a modified version of the relatively new technology for editing genes CRISPR / Cas9 was used. The technology for editing the CRISPR / Cas9 genes is associated with the use of an adeno-associated virus that helps deliver genetic material to its destination. Using this technology, successful experiments were carried out on editing the genes of individual cells in test tubes and unicellular embryos. Unfortunately, so far the possibility of genetic manipulation on human embryos causes violent disputes.
CRISPR / Cas exceeded all expectations. He allowed with the minimum number of errors both to “turn off” the necessary genes, and to embed new genes in strictly specific regions of the genome.
In December 2015, the scientific group Feng Janga modified this system so that it became completely infallible, which was published in the leading scientific journal Science. Scientists replaced the 3 amino acids “building blocks” of which the protein consists) in the endonuclease Cas9, after which the number of errors of such a system was reduced to almost zero.
The use of CRISP / Cas9 is especially important for gene therapy of aging, where it is necessary to influence the paths of longevity, common to most cells of the body. For gene therapy of aging, up to 2015, no human clinical trials have been carried out, which is not surprising, since aging has not yet been recognized as a disease.
In addition, gene therapy of aging is still very young and developing area. Now all research on gene therapy of aging is carried out on model mice, rats, monkeys and human cell cultures - cells in vitro.
All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where small RNAs are introduced that "turn off" the gene or the pathway of aging. That is, in the first case, something useful is introduced for longevity, and in the second, the harmful is turned off. In the strict sense, only two studies have been conducted on mammalian gene therapy of aging until 2015.
Much more work is modeling gene therapy on transgenic mice. In such studies, the therapeutic gene is not delivered into the body of an adult mouse, and with the help of genetic engineering they create mice whose genome has been changed since birth. Like gene therapy, it allows you to explore how an increase or decrease in the activity of different genes affects the lifespan and aging of the body.
Let's look at what can theoretically be done with the help of gene therapy and genetic engineering to fight aging.
The advantage of gene therapy over other ways of extending life
Why do we need gene therapy if you can use anti-aging drugs (geroprotectors)? Compared with other approaches to life extension (for example, geroprotectors or food restriction , prolonging life up to 30-50%), it is enough to carry out gene therapy only once in a lifetime, and you need to take pills all the time and not forget - otherwise the result will not be complete For example, in the work of Andrzej Bartke in 2001, food restriction extended the life of mice by 30% . However, mice consumed a low-calorie diet for up to 670 days in a row - that is, every day, for half of their lives! For most people, this is not very realistic. And in the experiment on gene therapy by Maria Blasco (to be discussed further in this article) in 2012, gene therapy with telomerase led to a slightly smaller effect - mice began to live 20% longer. However, in this work, the mice received only 1 injection of the drug into the blood for a lifetime in a rather old age!
Therefore, if we are talking about the transmission of research on the extension of life per person, gene therapy has an absolute advantage, because it does not reduce the quality of life because of the need for constant treatment - to follow a certain diet every day or to constantly use geroprotectors or other medicines. Also, gene therapy is highly targeted and therefore has the potential for fewer side effects.
In addition, drugs have limitations on bioavailability in various tissues and organs.
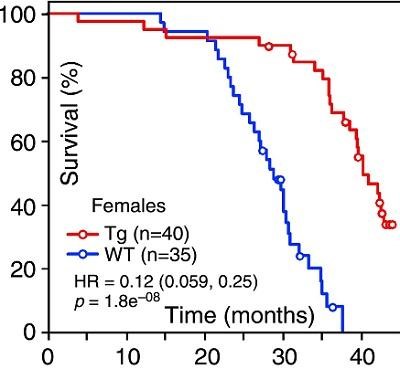
The introduction of the telomerase gene (TERT) in two-year-old wild-type mice (40-50 years old by human standards) with a single injection increases the length of telomeres and prolongs their life by 20%.

The history of telomerase research dates back to 1961. American researcher Leonard Hayflik cultivated human embryo fibroblasts in a test tube and noticed that they can share no more than 50 times, and then grow old. And if you take cells from older donors, then they divide even less.
The scientist suggested that in the cells there is a counter of divisions, limiting their total number. After 10 years, the Russian scientist Alexei Olovnikov offered a hypothetical mechanism for the operation of this counter.
Olovnikov suggested that during cell division, the ends of the chromosomes, called telomeres, are slightly reduced. And when telomeres reach a critical length, the cell stops dividing and ages. In the future, Elizabeth Helene Blackburn, an American cytogenetic scientist, won the Nobel Prize in Physiology or Medicine for 2009 jointly with Carol Grader and Jack Shostak with the phrase “for discovering the chromosome protection mechanisms of telomeres and the telomerase enzyme” according to the theory proposed by Alexey in 1971 Olovnikov.
In non-aging cells (for example, sex and embryonic stem), on the contrary, there must be an enzyme that extends telomeres, allowing the cells to divide almost indefinitely. In addition, it was shown that damage to the telomerase gene greatly shortens the life of model animals and leads to the emergence of premature aging syndrome - progeria.
After the discovery of telomerase, dozens of scientists caught fire in order to make a cure for old age based on it. It would seem that the "inclusion" of telomerase in all cells can make the body immortal. However, concerns soon arose due to the fact that the active synthesis of telomerase was observed in 90% of cancerous tumors. The question arose: will not the activation of telomerase lead to the risk of malignant transformation?
In addition, it turned out that cell aging is not always accompanied by a reduction in telomeres. For example, in the case of epithelial cells of the oral mucosa or cornea of the human eye. This suggested that telomerase activation alone may not be enough to rejuvenate the entire body. Before proceeding to gene therapy, the effects of telomerase were investigated in transgenic mice. It turned out that if you "turn on" the TERT gene in all cells of the mouse, then the lifespan is increased by 40%! However, the constant activity of telomerase increased the risk of cancer. Therefore, there was a question about how to activate telomerase work for a shorter period.

This was done in the work of Maria Blasco 2012 (see chart). The telomerase gene was delivered to the mouse using an adeno-associated virus (AAV9) capable of providing systemic delivery. Adeno-associated viruses are characterized by high safety: they do not embed the delivered gene into the host genome and therefore do not lead to mutagenesis (there is no risk of cancer). In addition, they almost do not cause an immune response. At the same time, TERT genome therapy was completely safe: the risk of cancer in mice did not increase. Two-year-old mice were given one injection, with adenovirus, to which the telomerase gene was inserted. This prolonged the life of the mice by 20% (as shown in the graph above). And this theoretically can allow people aged 40-50 years to give one injection of such a medicine and prolong their life by another 8-12 years.
Today, telomerase can be stimulated with drugs. An interesting study in this area was conducted by scientists from the University of Ljubljana (Slovenia) in 2016 after a series of successful clinical trials on vessel rejuvenation with low doses of valsartan and fluvastatin. This time they measured the activity of telomerase after vascular rejuvenation in the blood samples of 130 patient patients.
So a one-month course of valsartan 20 mg + fluvastatin 10-20 mg significantly increases the activity of telomerase by 3.28 times , which significantly correlates with the improvement of endothelial function (vascular rejuvenation) and reduction of inflammation in the blood vessels. And this increased level of telomerase persists, gradually decreasing, for another six months. But how effective such an increase in telomerase affects telomeres remains to be determined.
 It is important to know that telomeres may not necessarily prolong our life if such therapy is not done at the right time and for too long.
It is important to know that telomeres may not necessarily prolong our life if such therapy is not done at the right time and for too long.Moreover, telomerase stimulation alone can not lengthen telomeres. Telomerase activity hardly changes with age - look at the graph on the left. And the telomeres are still shrinking.
 Also today there is a drug on the market that increases telomerase activity - TA-65. He is very expensive, and the life of the mice did not prolong the research. Look at the graph on the left. In a study of 2011, scientists from the Spanish National Cancer Center began to give TA-65 long-lived two-year-old mice to increase telomerase, as in the previous study. Only in a previous study, mice were injected for gene therapy. But TA-65 did not prolong the life of mice, unlike gene therapy (see chart on the left), and turned out to be absolutely useless for prolonging life and slowing aging.
Also today there is a drug on the market that increases telomerase activity - TA-65. He is very expensive, and the life of the mice did not prolong the research. Look at the graph on the left. In a study of 2011, scientists from the Spanish National Cancer Center began to give TA-65 long-lived two-year-old mice to increase telomerase, as in the previous study. Only in a previous study, mice were injected for gene therapy. But TA-65 did not prolong the life of mice, unlike gene therapy (see chart on the left), and turned out to be absolutely useless for prolonging life and slowing aging.In 2011, scientists from the University of Texas studied telomeres and telomerase in cell cultures of more than 60 species of mammals . The role of telomeres in longevity was not so obvious ... Studies show (when comparing about 60 species of mammals), the longer the telomeres of a species are, the faster DNA mutations accumulate in it, the more cancers and shorter the lifespan. Telomere length is inversely correlated with longevity. This suggests that the result of telomerase life extension, which was obtained in mice with a single injection, may not prolong the life of people. The question on telomeres for people remains open.
Conclusion: In the future, theoretically, we can, through the introduction of the telomerase gene (TERT) at the age of 40-50 years with a single injection, increase the length of telomeres, but this therapy alone is not enough. Most likely, we must find a combination of gene-therapeutic effects in order to significantly prolong human life. Today we can imitate the effect with one-month therapy 1 time in half a year with a combination of drugs of valsartan 20 mg + fluvastatin 10-20 mg , or telmisartan + atorvastatin 10 mg. At least, these drugs in combination are able to stimulate telomerase itself.
Disruption of the Agtr1a gene, which encodes angiotensin receptor AT1a, prolongs the life of transgenic mice by 26% compared to wild-type mice.
Angiotensin II receptor antagonists, or AT1 receptor blockers, are one of the newer groups of antihypertensive drugs (drugs for treating blood pressure). Such drugs include all drugs of the Sartan group (for example, Telmisartan) .
As has been shown in studies , these drugs greatly prolong the life of animals and even humans. For example, according to a 2007 study conducted by scientists from the University of Buenos Aires (Argentina), one of the Sartans losartan, when given to 40 Wistar rats, he extended their maximum life expectancy by 18% compared to the other 40 rats in the control group .
 AT1 receptors can also be affected genetically by acting on the Agtr1a gene. This solves the problem of drug delivery to all tissues and organs. A unique study was conducted by researchers from the Institute of Pharmacological Research (Italy) and Duke University (USA) in 2009. As we can see on the graph, disruption of the Agtr1a gene encoding angiotensin receptor AT1a prolongs the life of 20 transgenic mice compared to 10 wild-type mice by 26%. Judging by research, functional aging of organs and tissues slowed down.
AT1 receptors can also be affected genetically by acting on the Agtr1a gene. This solves the problem of drug delivery to all tissues and organs. A unique study was conducted by researchers from the Institute of Pharmacological Research (Italy) and Duke University (USA) in 2009. As we can see on the graph, disruption of the Agtr1a gene encoding angiotensin receptor AT1a prolongs the life of 20 transgenic mice compared to 10 wild-type mice by 26%. Judging by research, functional aging of organs and tissues slowed down.This gene therapy can be used in the elderly to prolong life. The effect on AT1 receptors for people with high blood pressure is especially relevant.
Conclusion: In the future, using gene therapy, we can disrupt the gene encoding angiotensin receptor AT1 and prolong human life for 5-15 years (such dates are taken by extrapolating the results obtained in animals) depending on the patient's condition. Today, a partial effect (due to incomplete bioavailability in all tissues of the body) can be achieved by therapy of course treatments in low doses of the drug telmisartan at a dose of 10 mg per day , if there is no high blood pressure - otherwise the dose is higher.
Knockout of the gene encoding GHRKO growth hormone receptors (selective removal of growth hormone receptors) extends the life of transgenic mice by about 30% compared with wild-type mice.
 In humans, growth hormone regulates the level of IGF-1 (insulin-like growth factor 1 type). It happens like this. HGH is secreted in the anterior lobe of the pituitary gland. The growth hormone then binds to receptors in the liver. Due to binding to growth hormone receptors, insulin-like growth factor 1 type (IGF-1) begins to be secreted in the liver.
In humans, growth hormone regulates the level of IGF-1 (insulin-like growth factor 1 type). It happens like this. HGH is secreted in the anterior lobe of the pituitary gland. The growth hormone then binds to receptors in the liver. Due to binding to growth hormone receptors, insulin-like growth factor 1 type (IGF-1) begins to be secreted in the liver.If growth hormone receptors are not blocked or “broken,” the higher the growth hormone, the higher the IGF-1. Today, one of the most proven in almost all animal species ( www.karger.com/Article/Abstract/212538 ; www.ncbi.nlm.nih.gov/pubmed/19590001 ) the life extension method is the reduction of IGF-1 (insulin is a similar factor type 1 growth) with the help of fasting , or with the help of the FMD diet, developed by the scientist Walter Longo from the University of Southern California (USA) in 2015.
But for this you need to follow a diet all your life, which not everyone can do.
IGF-1 is a hormone that binds to IGF-1 receptors. And so, if the IGF-1 receptors are genetically disturbed, then the IGF-1 deficiency can be partially imitated.
According to a 2008 study made by scientists from the Albert Einstein Institute (USA), a violation in the genes encoding receptors for IGF-1 in 197 Jewish descendants of Ashkenazia's long-livers (105 women and 92 men) probably explains their longevity phenomenon. It seems that superdolongers, who live for more than 100 years, had an optimal (not as high as other people) level of IGF-1 in childhood and adulthood, or IGF-1 had a high level, but at the same time a genetically determined defect of receptors to IGF-1.
What if IGF-1 optimization and mortality reduction is just a coincidence. After all, the longevity is not so much.We would be told about such a relationship by research on large groups of people. There are such studies.

Research data suggests that there is an IGF-1 level that is higher and lower than the risk of death from cancer and other causes in humans.
This range is from about 105 ng / ml to 120 ng / ml. (see the chart on the left). This fact was shown in their studies by Swedish scientists from the University of Gothenburg in 2012 as a result of a study of 2901 patients, as well as by American scientists from the Gerontology School of Davis at the University of Southern California in 2014. In people aged 50 to 65 years, IGF-1 at a level of 105 to 120 ng / ml was associated with the lowest total mortality in the next 18 years.
 Knockout in mice of the gene encoding GHRKO growth hormone receptors (selective removal of growth hormone receptors) prolongs the life of transgenic miceby about 30% compared with wild-type mice.
Knockout in mice of the gene encoding GHRKO growth hormone receptors (selective removal of growth hormone receptors) prolongs the life of transgenic miceby about 30% compared with wild-type mice.On the chart: the black color of the line is a wild type mouse. The blue chart is wild-type mice that were undernourished to reduce their IGF-1 (they lived 30% longer than wild-type mice).
The red line is a mouse with a genetically low IGF-1, since they have knocked out the gene encoding the GHRKO growth hormone receptors. They lived 30% longer than wild-type mice).
The green line is the same mouse with a knockout of the gene encoding the GHRKO growth hormone receptors, but which are also under-nourished - their under-feeding did not give any advantages , -1 . ? , -1 , , .
 , , .
, , .GHR-KO 11C, (Andrzej Bartke) — , ().
6 , 1819 . - , -1.
25 2003 Longevity Prize — . , -1 500 .
60 , -1 30 3-4 (500 3 ).
: , GHRKO ( ), , -1 5-17. , . , -1 2- 500 .
FGF21 ( 21) .
 Fibroblast growth factor 21 (FGF21) is a hormone secreted by the liver during fasting periods in order to help the body adapt to nutritional deficiencies.
Fibroblast growth factor 21 (FGF21) is a hormone secreted by the liver during fasting periods in order to help the body adapt to nutritional deficiencies.FGF21 is also synthesized by the liver in response to diabetes and many other health problems, and even with alcohol. FGF21 blocks growth hormone receptors due to which the synthesis of IGF-1 by the liver is reduced.
Lower than usual IGF-1 in many studies has greatly prolonged the life of animals and reduced mortality in humans. It would be interesting to check whether it is possible to simply genetically force the body to secrete more FGF21. Will it prolong life significantly? As it turned out - you can.
By the way, Metformin is able to simulate the effect of increasing FGF21 at a dosage of 500 mg per day.
2013 () , FGF21 .
 () 2012 , FGF21 ( 21) ( ) ( ), .
() 2012 , FGF21 ( 21) ( ) ( ), .: FGF21 ( 21) . FGF21 Metformin at a dosage of 500 mg per day. But it is fatally dangerous to use metformin to people with elevated above normal creatinine in blood tests. Therefore, before use, the doctor is obliged to prescribe a blood test for creatinine. Otherwise, metformin is considered a drug with a high safety profile.
Genetic knockout Adenylate Cyclase type 5 prolongs the life of transgenic mice by 30% compared with wild-type mice.
, . , , , .
— — . . AC5 ( www.ncbi.nlm.nih.gov/pubmed/18205980 ; www.ncbi.nlm.nih.gov/pubmed/19922557 ). (, ).
It should be noted that it is not possible to combine the genetic blockade of growth hormone receptors and Adenylate cyclase type 5 (AC5). As shown in a 2013 study (University of Medicine and Dentistry of New Jersey - USA), mice in such an experiment died within a month, as if they were starving. These mice were blocked with AC5 and reduced calorie intake. Therefore, either starve or completely block the AC5. Either starve and reduce the excessive activity of AC5, but not block it completely.
— , - («flight or fight»); , , , , , . , , , .
Stephen F. Vatner «» 5 (AC5) — , — β-.
 Such receptors are present on almost all cells of the body. Look at the chart. The knockout of AC5 greatly extended the median lifespan of transgenic mice by 30% compared with wild-type mice, protected the heart from aging, protected it from type 2 diabetes and obesity.
Such receptors are present on almost all cells of the body. Look at the chart. The knockout of AC5 greatly extended the median lifespan of transgenic mice by 30% compared with wild-type mice, protected the heart from aging, protected it from type 2 diabetes and obesity.At the molecular level, these effects were due to the fact that the inactivation of AC5 launched the path of resistance to stress - Raf / MEK / ERK.
As a result, the cell produced a whole set of protective molecules, triggering the mechanisms of cell survival. In addition, it is known from earlier works that the action of adrenaline on a cell through β2 -adrenoreceptors directly causes DNA damage.
Thus, it can be assumed that "turning off" the AC5 gene of mice also contributed to increased genome stability.
, AC5 , . FDA -9-β-- (Vidarabine AraAde), 5 .
, 5 — , .
, , 5 .
, — . 1988 .
Kaplan, using the example of primates, showed that if a group of primate males was assembled, then for some days the monkeys would have a social hierarchy. The worst place in such a hierarchy is below. Primate males in a subordinate position show a number of indicators of chronic stress. Often they develop atherosclerosis. When scientists gave primate males that are at the bottom of the social hierarchy (risk group), beta-blocker propranolol , which suppresses the activity of the sympathetic nervous system, atherosclerosis of the vessels did not develop.
It turned out that the sympathetic nervous system negatively affects the development of atherosclerosis and is involved in problems with the heart and blood vessels due to stress. Emotional stress realizes itself through the sympathetic (adrenergic) autonomic nervous system, which connects the control centers of our brain and internal organs. Including - with the immune, bone marrow and others. Atherosclerosis is the main factor that leads to the greatest number of deaths in developed countries from heart attack and heart stroke.
A randomized, double-blind, placebo-controlled study in 1983 , organized by Goldstein S and colleagues, showed that propranolol therapyin 3837 patients with acute myocardial infarction reduces mortality from cardiovascular diseases (cause of death # 1 in the world).
A three-year observation of 60-69 years old patients from the United States who suffered a myocardial infarction, published in 1997 , showed that propranolol reduced overall mortality in such patients by 35-37%. In addition, propranolol caused a decrease in signs of disease and aging of the heart (a significantly greater increase in the left ventricular ejection fraction and a significantly greater decrease in the mass of the left ventricular myocardium).
Conclusion: , 5- . , , , , -. , , . , , , !!! 5 ( 50%) in mini-doses, significantly lower than high doses used in the treatment of cognitive disorders.
Removal of RIP3 (serine / threonine protein kinase 3 receptor interaction) extends the life of atherosclerotic mice by 30% to the level of healthy wild-type mice.
, - , . , , ( ) , , .
«−/−» — . — . ( ) . , , . , ,
[www.ncbi.nlm.nih.gov/pmc/articles/PMC4066890].
 Apoe . 2015 , , . , , ApoE +/+ — ApoE, . . ApoE -/-, . ApoE -/- RIP3 (RIP3-/-), , , ApoE +/+ .
Apoe . 2015 , , . , , ApoE +/+ — ApoE, . . ApoE -/-, . ApoE -/- RIP3 (RIP3-/-), , , ApoE +/+ ., ApoE -/- - RIP3 +/+ 25 . RIP3 (RIP3 -/-), 32 — .
RIP3 , . . — , .
RIP3 , , .
50% ApoE -/- RIP3 +/+ , ApoE -/- RIP3 -/- 88% .
RIP3 NF-κb , ( ). : RIP3 +/+ . RIP3 () .
From the previous data, the reader can conclude that after RIP3 knockout, although atherosclerosis will not develop, there will be a danger of cytomegalovirus. After all, RIP3 causes apoptosis (self-destruction) to protect against viral infection. Human cytomegalovirus (CMV) is a major virus that causes birth defects and serious problems in immunocompromised persons. CMV is also associated with atherosclerosis (vascular disease). Previous studies have shown that the induction of mTOR independent autophagy can block the replication of various DNA and RNA viruses.
In 2015, scientists from the University of California (USA) on cell lines proved that mTOR activation of independent autophagy with trehalose could be another method for treating CMV. Same resultswere obtained in 2008 at the University of Paris (France). As an mTOR inducer of independent autophagy, trehalose also showed itself in clinical trials. Now there is the relevance of conducting clinical trials of intravenous trehalose for the treatment of CMV. Trehalose is a sugar that can enter our regular diet instead of sugar, as it is a potential geroprotector (a means of prolonging life).
 By tradition, let's see if it is possible to crush RIP3 with the means available to us today. It turns out you can. So, according to a 2016 study by scientists from Peking University, low doses of aspirin (L-ASA), though not reducing necrosis through RIP3 to the level of healthy mice (control), reduce necrosis at least partially compared to SAP. Look at the chart.
By tradition, let's see if it is possible to crush RIP3 with the means available to us today. It turns out you can. So, according to a 2016 study by scientists from Peking University, low doses of aspirin (L-ASA), though not reducing necrosis through RIP3 to the level of healthy mice (control), reduce necrosis at least partially compared to SAP. Look at the chart.0,2. — 0,8. — 0,3 ( , ).
, , ? 2002 , , , , .
- [ www.ncbi.nlm.nih.gov/pubmed/16326168 ]
- [ www.ncbi.nlm.nih.gov/pubmed/26843277 ]
- [ www.ncbi.nlm.nih.gov/pubmed/25824964 ]
RIP3 (necrosulfonamide).
: RIP3 . , , RIP3 : 250 1 .
PCSK9 , .
Cardiovascular diseases are the number one killer worldwide. In June 2014, scientists at Harvard University were able to edit the genes in> 50% of the liver cells of an adult mouse - using adenovirus with Cas9 / CRISPR.
It was possible to make changes in the PCSK9 gene using a single intervention. People with such changes in this gene have lower levels of bad cholesterol and are less likely to suffer from cardiovascular diseases. Thus, it was shown that only one injection can reduce the level of bad cholesterol and in the future do without drugs to reduce the level of cholesterol - statins .
Genetic overexpression of Klotho increases the lifespan of transgenic mice by 19-30% compared with wild-type mice.
Twenty years ago, in 1997, Japanese and American scientists, led by Yo-ichi Nabeshima, discovered a new longevity gene and named it in honor of the Greek goddess of fate, the weaving thread of life - Klotho. His "off" in transgenic mice caused accelerated aging syndrome.
After 7 years, the same researchers found that the activation of klotho significantly prolongs the life of mice - males by 20-30%, and females - up to 19%.
Klotho is produced in the kidneys and blood vessels of the brain and is carried by the blood through the body like a hormone. It systemically affects most cells in the body - binds to receptors on their surface and inhibits insulin and IGF-1 signaling (one of the main ways of aging).
Three years after the discovery of the klotho gene, in 2000, the University of Gumma in Japan carried out the first work on gene therapy , but not from aging, but from chronic vascular disease, atherosclerosis. Ryozo Nagai and colleagues systematically introduced this gene to rats and achieved improved vascular status and lower blood pressure.
In 2014, a joint study of Japanese and American scientists from the Jichi Medical University, as well as the University of California and Boston showed that certain variants in the klotho gene (SNP Rs9536314) are associated with longevity and high mental abilities.
 To understand how Klotho influences us, consider a study conducted in 2005 by scientists from the University of Texas (USA), which showed that mutagenic mice with a Klotho gene with a defect looked normal for about 3-4 weeks of age and then began to age fast.
To understand how Klotho influences us, consider a study conducted in 2005 by scientists from the University of Texas (USA), which showed that mutagenic mice with a Klotho gene with a defect looked normal for about 3-4 weeks of age and then began to age fast.For a month, they showed such bright signs of aging: skin atrophy, osteoporosis, atherosclerosis, and emphysema. About two months later, the mice died.
 In other studies, scientists from the University of Texas (USA) have bred a second type of mutagenic mouse , in which the Klotho gene produces significantly more protein than normal mice. These mice lived on 19% - 30% longer than their usual relatives (see the graph on the left).
In other studies, scientists from the University of Texas (USA) have bred a second type of mutagenic mouse , in which the Klotho gene produces significantly more protein than normal mice. These mice lived on 19% - 30% longer than their usual relatives (see the graph on the left).Scientists note that Klotho is the rarest case in mammalian biology, where a single gene has such a significant effect on life expectancy. Overexpression of the klotho protein causes insulin resistance to IGF-1, imitating starvation.
Scientists from the University of California (USA) in 2015 showed that the level of klotho protein naturally decreases as the body ages, which leads to a deterioration in cognitive abilities. Increased expression of the klotho protein is associated with better cognitive functions in normal healthy people.
While we are not able to increase the level of klotho in the body with the help of genetic engineering, we can do this with the help of drugs. A randomized controlled study on people aged about 60 years , published in 2015 by the Royal College of London (United Kingdom) in the journal CJASN, showed that treatment with valsartan or losartan (drugs for high blood pressure) increases klotho longevity protein expression.
And in 2010, a study of the Nagoya University (Japan) was published in the American Journal of Nephrology , where it was shown how activated carbon increases the expression of klotho longevity protein in rats. Activated carbon inhibits kidney overload with indoxyl sulfate, which leads to an increase in kloto. Indoxyl sulfate is the product of tryptophan metabolism in the intestine using microflora to indole and further metabolism in the liver to indole sulfate. But activated carbon can adsorb indole sulphate and intestinal bacteria that are involved in the metabolism of other kidney toxins. Indole has a toxic effect on the cells of the kidneys and blood vessels - increasing oxidative stress in the kidneys and in the vessels. As the glomerular filtration rate of the kidneys decreases with aging, the renal clearance of indoxyl sulfate decreases in humans and in rats, which leads to its increased content, toxicity and, consequently, to a decrease in klotho. Indoxyl sulfate is a uremic toxin that accelerates the progression of chronic renal failure.
Conclusion: In the future, we may be able to increase Klotho expression and prolong life with the help of genetic engineering, depending on the time of the intervention and gender. Today, some blood pressure medications ( valsartan and losartan ) have been shown to increase klotho in humans, and also increase the use of activated charcoal , at least in rodents.
Genetic receptor knockout of RAGE glycation end-products can potentially prolong life in the same way as fasting and calorie reduction.
The accumulation of glycation end products (AGE) is one of the causes of human aging, as well as many age-related diseases: atherosclerosis, myocardial infarction, congestive heart failure, diabetic retinopathy, diabetic neuropathy, diabetic nephropathy, Alzheimer's disease, psoriasis. RAGE is a receptor that binds to the final glycation products (AGE). The binding of AGE and this receptor (RAGE) leads to the formation of reactive oxygen species (ROS) with the subsequent activation of the oxidatively sensitive NF-κB transcription factor in the vascular wall and not only regulating the expression of inflammatory and “responding to damage” genes, and directly to the RAGE gene . The negative effect of AGE products is realized not only in the walls of the vessels, but also in neurons and even in bone tissue.
In 2014, thanks to scientists from George Mason University, it became clear that birds do not have RAGEs , and it is possible that they have a longer lifespan at higher body temperature and blood glucose levels than mammals of the same mass.
Birds do not suffer from diabetes in normal conditions. In addition, the plasma of birds contains a lower concentration of methylglyoxal. Methylglyoxal is the precursor to the end products of glycation.
 A 2007 study on mice (Icana School of Medicine, Mount Sinai Medical Center, USA) showed that a diet with a reduction in mice of one of the most common end products of glycation (carboxymethyl-lysine) from about 30 weeks old (from youth humans) was sufficient to significantly increase the average and maximum life expectancy of animals (by 15% and 6%, respectively).
A 2007 study on mice (Icana School of Medicine, Mount Sinai Medical Center, USA) showed that a diet with a reduction in mice of one of the most common end products of glycation (carboxymethyl-lysine) from about 30 weeks old (from youth humans) was sufficient to significantly increase the average and maximum life expectancy of animals (by 15% and 6%, respectively).The graph shows that mice with a reduction in the diet of the end products of glycation lived much longer. In addition, in mice with low levels of glycation end products in the diet, body weight was significantly reduced. This shows that excess weight is not only a consequence of calories in food, but also a consequence of a high consumption of glycation end products.
 However, as shown in the graph on the left (from a 2008 study by scientists from the University of Miami - USA), if in low-calorie diets in mice provide the same amount of glycation end products as in normal diets, then life extension is not observed.
However, as shown in the graph on the left (from a 2008 study by scientists from the University of Miami - USA), if in low-calorie diets in mice provide the same amount of glycation end products as in normal diets, then life extension is not observed.But you can not reduce the end products of glycation, but simply knock out with the help of genetic engineering RAGE receptors. Genetic blockade of RAGE receptors in mice has been carried out more than once by researchers from Brigham Young University (USA) in 2016, as well as in the same year by Swedish scientists from Uppsala University . Such blockades led to positive health outcomes for mice. How this will affect the prolongation of the life of the mice apparently has not been verified.
Translated into our diet - it will look like this. If you reduce the calories, but at the same time eat fried, baked food, as well as a lot of sweets and flour, then there will be no health benefits.
At the same time, even if you do not reduce calories in the diet, but completely exclude fried, baked meat from the diet, eat meat only in boiled form, and vegetables only in cheese, do not eat sweets and foods with a high glycemic index, add means inhibitors of final products glycation ( metformin 500 mg per day, vitamin B6 5-20 mg per day, raw broccoli cabbage 100 grams per day), it is possible to increase the lifespan. According to Japanese scientists from Kurume University in 2008, telmisartan, also a drug, reduces the ability of RAGE to bind to the end-products of glycation in hypertensive patients.
Genetic overexpression of BubR1 protects DNA, increases the maximum lifespan of transgenic mice by 15% and significantly reduces the incidence of cancerous tumors even in the presence of DNA mutations.
 The researchers found that a protein called BubR1 reduces the risk of cancer and by 15% and the maximum lifespan of experimental mice.
The researchers found that a protein called BubR1 reduces the risk of cancer and by 15% and the maximum lifespan of experimental mice.BubR1 protein helps cells form chromosomes in the process of division. When BubR1 expression is reduced, chromosomes can not properly divide during mitosis, and cells with defective chromosomes are formed.
BubR1 protein is studied superficially, but it is known that most cancer cells are formed precisely because of defective chromosomes, or rather, their different numbers. This phenomenon is called aneuploidy (a change in karyotype, in which the number of chromosomes in cells is not a multiple of the haploid set), and it is common to all cancer cells, although it is not known for certain whether aneuploidy is the main cause of cancer.
 However, aneuploid cells have too few or too many chromosomes, and BubR1 protein plays a key role in this phenomenon.
However, aneuploid cells have too few or too many chromosomes, and BubR1 protein plays a key role in this phenomenon.In the course of the experiments, in 2013, American scientists from the Mayo Clinic in Rochester, Minnesota, bred using transgenic mice (T23) with the increased expression of BubR1 by genetic engineering .
Then the experimental mice were subjected to the action of irreversibly causing tumors of the lungs and skin.
As a result, it turned out that oncological diseases “earned” only 33% of transgenic mice (T23) with over-expression of BubR1.
In this case, the disease in mice with hyper-BubR1 occurred later than in wild-type mice - after about 2 years, and the death rate from cancer was several times lower - 15% versus 40% (see the first graph on the left). Moreover, the life expectancy of rodents increased by 15% (see the second graph on the left), and their physical form impressed even scientists. So, the mice ran through the treadmill 200 m, and not 100, like normal mice.
Perhaps Van Dersen and his colleagues took the first step toward creating new cancer treatments and ways to delay aging. Interestingly, in 2005, scientists from the University of Pennsylvania (USA) showed that the antibiotic doxycycline , which is seen as a powerful drug for the treatment of certain types of cancer, can increase the expression of BubR1.
Conclusion: In the future we will be able to increase the expression of BubR1 with the help of genetic engineering and prolong the life of years by 10, as well as delay the development of many types of cancer. Today in clinical trials, the antibiotic doxycycline , which is a potent inhibitor of cancer cell proliferation in animals, is being tested for the treatment of certain types of cancer. And doxycycline, is just the activator of BubR1.
Genetic overexpression of MTH1 increases life expectancy in transgenic mice by 22% compared with wild-type mice.
A characteristic feature and, apparently, one of the main causes of aging is the accumulation of errors and DNA mutations. In addition, numerous diseases of premature aging - progeria - also arise as a result of excessive accumulation of genomic damage.
During the life of the DNA is constantly damaged by various influences. From the external environment it is affected by temperature, UV radiation, chemical mutagens and viruses. But the internal processes of the cell itself increase the genomic instability: DNA is damaged by active forms of oxygen from mitochondria, and replication systems (DNA doubling) and DNA repair often work inaccurately and introduce errors themselves.
As a result, with age, a whole spectrum of disorders accumulates in the DNA: breaks, point mutations, transfer of pieces of DNA from one place to another, sticking of chromosomes, inserting viruses and transposons into the genome. If there is too much damage, the cell is forced to go in one of 3 ways: start the processes of cell suicide (apoptosis), become senescent or turn into cancer. All these processes (if they are subject to many cells) are extremely harmful to the body.
The most studied way to increase the stability of the genome is the activation of DNA repair systems. This approach is used in most of the work on gene therapy.
 In 2013, Italian scientists led by Gemma Calamandre investigated transgenic mice with over-expression of the MTH1 gene . Protein MTH1 is involved in the repair of DNA damage and RNA caused by reactive oxygen species.
In 2013, Italian scientists led by Gemma Calamandre investigated transgenic mice with over-expression of the MTH1 gene . Protein MTH1 is involved in the repair of DNA damage and RNA caused by reactive oxygen species.It corrects defective nucleotides - guanidines oxidized at the eighth carbon atom (of the four letters on which this DNA is written is denoted by “G”). It turned out that increased expression of MTH1 prolongs the life of transgenic mice by up to 22% (see graph).
In addition, in these mice, genomic damage accumulated with age much less. Their behavior also changed: the mice became less disturbing and more curious.
Genetic engineering is not available in humans today. Nevertheless, according to 2014 data from Japanese scientists at Tottori University, we can slightly affect repair and protect DNA and increase MTH1 with the help of magnesium. One of the best ways to maintain the necessary level of magnesium in the blood is supplementation of magnesium citrate 300-400 mg of elemental magnesium per day.
French scientist Elena Martin and her colleagues in 2008 demonstrated in their study that magnesium is important for the functioning of many enzymes involved in copying and restoring DNA damage. Chronic magnesium deficiency in food leads to oxidative stress and accumulation of DNA damage, which was also shown by scientists from Duke University (USA) in 1988, scientists from the University of Karlsruhe (Germany) in 2001.
When examining patients from the University Hospital San Juan de Reus (Spain) in 2014, it was revealed that people with the highest levels of magnesium in the plasma within the normal range and the highest consumption of it, died 34% less often - that is, they had 34% lower mortality from all causes.
Conclusion: In the future, with the help of genetic engineering or gene therapy, we will be able to increase the expression of MTH1 and prolong life for 6-12 years, protecting DNA from damage. And today, to partially achieve the same effect, we can use magnesium citrate 300-400 mg as supplements. Moreover, magnesium showed a decrease in mortality in humans by 34%.
Conclusion
There is a lot of evidence that the impact on several paths of longevity at once gives a greater effect on life expectancy than the impact on them separately.
An extraordinary survivor - a naked digger - a rodent that lives 10 times longer than a mouse, differs from it by activating a whole set of longevity pathways: this is increased resistance to stress, protection against cancer, stability of protein molecules, protection against neurodegeneration.
Thus, in order to develop a gene therapy that drastically prolongs life, it is necessary to act simultaneously on several molecular paths.
It is necessary to combine those gene therapy approaches that are being developed now to increase the stability of the genome, rejuvenate the hypothalamus, destroy senescent cells, increase resistance to stress, improve mitochondrial function, and maintain stem cell niches. It is important to remember that not individually tested combination of effects in studies useful mechanisms can shorten life.
If now single-gene therapy does not lead to an increase in life expectancy of more than 20-30%, then we hope that in the future, due to the complex therapy of aging, it will be possible to achieve truly fantastic results.
Laboratories in the USA , laboratories in China and the UK have announced plans or ongoing research on the use of the crispr genome editing technology for human embryos. True, so far these attempts have failed. But it all starts with trial and error.
On August 30, 2017, the United States Food and Drug Administration (FDA) approved the world's first blood cancer gene therapy .
In March 2017, French scientists reported on successful clinical studies of gene therapy for the treatment of sickle cell anemia.
The Committee of the American National Academy of Sciences and the National Academy of Medicine gave support for editing the human embryo genome as early as 2017 . But only for serious diseases and under strict control.
findings
1. All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where the gene is turned off or the path of aging.
2. Compared with other approaches to the extension of life, it is enough to carry out gene therapy only once in a lifetime.
3. Introduction of the telomerase gene (TERT), disruption of the Agtr1a gene, GHRKO knockout, disruption in the genes encoding IGF-1 receptors, over FGF21 expression, AC5 knockout, RIP3 deletion, PCSK9 gene editing, over Klotho expression, over RAGE knockout, over BubR1, MTH1 — , .
4. , .
5. : , , , 6, , , + , , FMD, , , , , .
Source: https://habr.com/ru/post/370981/
All Articles