What a disease: cancer. Molecular Diagnosis
We are completing a series of articles on cancer.
Today, the Atlas will explain in detail what molecular testing is and how it affects the diagnosis.

In the photo: Vladislav Mileyko, Head of Oncodiagnostics ,
biomedical holding "Atlas".
To understand how molecular diagnostics works and what place it occupies in oncology, you must first understand the mechanisms occurring in the tumor.
Every healthy cell contains a DNA molecule, from which it reads information, what form and specialty to get, what proteins and enzymes to produce, and most importantly, when to divide and die. Read more about how this happens in our first article .
')
Mutations in proto-oncogenes and suppressor genes, which are responsible for cell division and death, cause the cell to stop following instructions and to synthesize proteins and enzymes incorrectly. Molecular processes are out of control: the cell is constantly dividing, refuses to die and accumulates genetic and epigenetic mutations. Therefore, malignant neoplasms are often called genome diseases.
Hundreds of thousands of mutations can occur in tumor cells, but only a few of them contribute to the growth, genetic diversity and tumor development. They are called driver. The remaining mutations, “passenger” (passanger), by themselves do not make the cell malignant.
Driver mutations create different cell populations, which provides a variety of tumors. These populations or clones react differently to treatment: some of them are resistant and lead to relapse. In addition, the different sensitivity of clones to therapy can lead to a radical change in the molecular profile during treatment: even small cells at the beginning of a population can gain an advantage and become dominant at the end of treatment, which will lead to stability and tumor development.

Source: Cell .
Illustrations: Michael Kowalski .
Driver mutations, changes in the amount or structure of proteins are used as biomarkers - targets for which the treatment is selected. The more targets that are known, the more accurate can be the choice of potentially effective treatment regimens.
Separating driver mutations from the rest and determining the molecular profile of a tumor is not easy. For this purpose, the technology of sequencing, fluorescent in situ hybridization (FISH), microsatellite analysis and immunohistochemistry is used.
Methods of sequencing the new generation allow to identify driver mutations, including those that make the tumor sensitive to targeted therapy.
With the help of FISH technology, tint regions of chromosomes on which a certain gene is located. Two connected multicolored dots are a chimeric or fused gene: when, as a result of chromosome rearrangement, sections of different genes are joined together. This can lead to the fact that the oncogene will fall under the influence of the regulation of another more active gene. For example, the fusion of the EML4 and ALK genes is of key importance in the case of lung cancer. The ALK proto-oncogene is activated under the influence of its “partner” on reorganization, which leads to uncontrolled cell division. Given the restructuring, the oncologist may apply a medicine that will be directed against the activated product of the ALK gene (Chrysotinib).
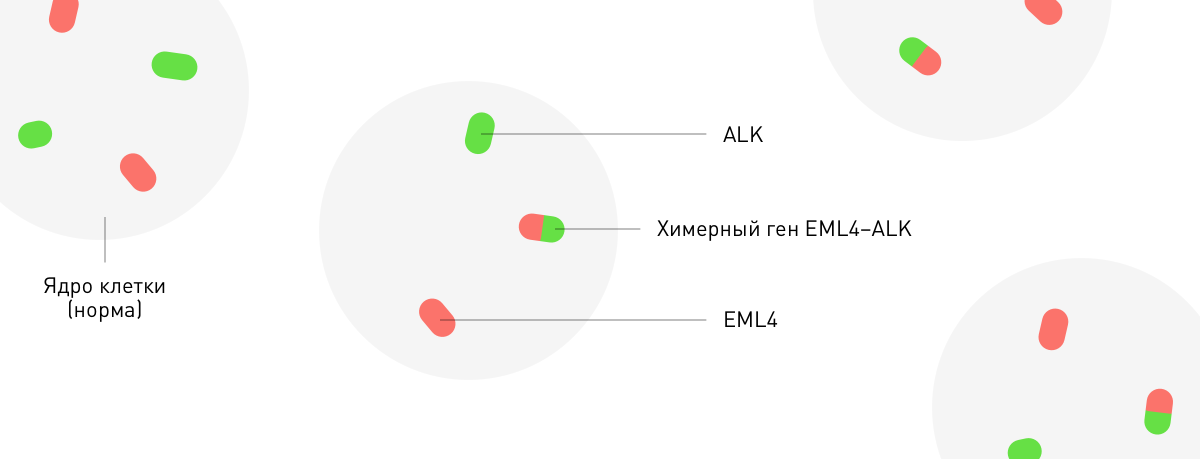
In situ Fluorescence Hybridization (FISH).
Microsatellite analysis shows the degree of violation of the DNA repair system, and immunohistochemistry - protein biomarkers located on the surface, in the cytoplasm and nuclei of tumor cells.
All these studies are part of the new product of the biomedical holding Atlas, the Solo test. Using this test, the oncologist receives information about the molecular profile of the tumor and how it affects the potential efficacy of a wide range of anticancer drugs.
Solo specialists are researching up to 450 genes and biomarkers to assess how a tumor can respond to the use of more targeted drugs for the treatment of cancer. For some of them, biomarker analysis is dictated by the manufacturer. For others, they use data from clinical studies and recommendations from the international community of oncologists.
In addition to the choice of targets for targeted therapy, molecular profiling helps to detect mutations that, on the contrary, make the tumor resistant to a specific treatment, or genetic features that are associated with increased toxicity and require individual dose adjustment.
For research, biopsy material or paraffinized blocks of postoperative material are used.
Molecular profiling provides additional information about the disease, but it is not always applicable to the choice of treatment. For example, in situations where standard therapy is sufficiently effective or surgical treatment is indicated. Clinical situations can be identified when such a study may be most useful:
Solo project specialists advise oncologists or patients and suggest whether a test is needed in this case.
The result of the diagnosis also includes recommendations for clinical studies with a suitable test drug. The patient has the opportunity to take part in them.
Usually in medical practice, general strategies are used to treat patients with a definite diagnosis. For small cell lung cancer, one strategy is used, for non-small cell, another. For cancer, this method is not always suitable. Due to differences at the molecular level, even with the same type of tumor, patients can receive ineffective or unnecessary treatment.
With the increase in research and the invention of targeted drugs, the approach to the treatment of cancer has begun to change. To increase the relapse-free period and the patient's life expectancy, the molecular profile of the tumor, the body's response to drugs and chemotherapy (pharmacogenomics) must be taken into account, and the main biomarkers must be known.

Precision medicine can significantly improve the prognosis of a particular patient, avoid serious side effects of cancer drugs and significantly improve the quality of life of the patient. But this method has drawbacks.
Targeted drugs are becoming more and more, and they have two main limitations: most molecular-targeting agents provide only partial suppression of signaling pathways and many of them are too toxic to be used in combination.
Imagine that you are an architect in Moscow. You face a difficult task - to solve the problem of traffic jams at rush hour by building one bridge. Molecular mechanisms can be compared with the movement of machines, and the bridge - the main drug, which should solve the main problem. It seems that several drugs (a series of bridges) aimed at major molecular disorders can solve this problem. But the toxicity of drugs increases and may be unpredictable.
We began to better understand the molecular processes of malignant tumors, but current methods of introducing accurate oncology into clinical practice are lagging far behind. To speed up the study of targeted therapy, scientists have developed two new approaches - Basket and Umbrella.

The essence of the Basket method is that patients with a specific biomarker are selected for the study, regardless of the location and name of the tumor. In May 2017, the FDA approved such a treatment for a biomarker called high microsatellite instability (MSI-H) or a defect in inconsistency recovery (dMMR).
Molecular disorders differ not only in different patients, but also within the same tumor. Heterogeneity is a big problem in oncology, to solve which the design of the study Umbrella was developed. For the Umbrella method, patients are first selected according to the type of malignant neoplasms, and then genetic mutations are taken into account.
Such studies help not only to collect information on the action of targeted drugs - sometimes this is the only way for patients who do not respond to standard treatment with registered drugs.
We decided to give an illustrative example of how the use of extended molecular profiling might look like.
A patient with melanoma of the skin and metastases in the liver turned to an oncologist. The doctor and patient decided to do molecular profiling to get more complete information about the disease. The patient underwent a biopsy and sent tissue samples for examination. As a result of diagnosis, several important genetic disorders were found in the tumor:
Focusing on the results of clinical studies and recommendations we can come to the following conclusions:
Thus, the doctor gets the opportunity to navigate among the possible treatment options based on not only the clinical parameters of the patient, but also taking into account the molecular characteristics of the tumor.
Molecular diagnosis is not a panacea for all oncological diseases. But it is an important tool for the oncologist, which allows you to approach the treatment of malignant tumors from the new side.
Thank you for reading and commenting on our oncology materials. Here is the full list of articles:
Today, the Atlas will explain in detail what molecular testing is and how it affects the diagnosis.

In the photo: Vladislav Mileyko, Head of Oncodiagnostics ,
biomedical holding "Atlas".
To understand how molecular diagnostics works and what place it occupies in oncology, you must first understand the mechanisms occurring in the tumor.
Molecular processes in the tumor
Every healthy cell contains a DNA molecule, from which it reads information, what form and specialty to get, what proteins and enzymes to produce, and most importantly, when to divide and die. Read more about how this happens in our first article .
')
Mutations in proto-oncogenes and suppressor genes, which are responsible for cell division and death, cause the cell to stop following instructions and to synthesize proteins and enzymes incorrectly. Molecular processes are out of control: the cell is constantly dividing, refuses to die and accumulates genetic and epigenetic mutations. Therefore, malignant neoplasms are often called genome diseases.
Hundreds of thousands of mutations can occur in tumor cells, but only a few of them contribute to the growth, genetic diversity and tumor development. They are called driver. The remaining mutations, “passenger” (passanger), by themselves do not make the cell malignant.
Driver mutations create different cell populations, which provides a variety of tumors. These populations or clones react differently to treatment: some of them are resistant and lead to relapse. In addition, the different sensitivity of clones to therapy can lead to a radical change in the molecular profile during treatment: even small cells at the beginning of a population can gain an advantage and become dominant at the end of treatment, which will lead to stability and tumor development.

Source: Cell .
Illustrations: Michael Kowalski .
Molecular Diagnosis
Driver mutations, changes in the amount or structure of proteins are used as biomarkers - targets for which the treatment is selected. The more targets that are known, the more accurate can be the choice of potentially effective treatment regimens.
Separating driver mutations from the rest and determining the molecular profile of a tumor is not easy. For this purpose, the technology of sequencing, fluorescent in situ hybridization (FISH), microsatellite analysis and immunohistochemistry is used.
Methods of sequencing the new generation allow to identify driver mutations, including those that make the tumor sensitive to targeted therapy.
With the help of FISH technology, tint regions of chromosomes on which a certain gene is located. Two connected multicolored dots are a chimeric or fused gene: when, as a result of chromosome rearrangement, sections of different genes are joined together. This can lead to the fact that the oncogene will fall under the influence of the regulation of another more active gene. For example, the fusion of the EML4 and ALK genes is of key importance in the case of lung cancer. The ALK proto-oncogene is activated under the influence of its “partner” on reorganization, which leads to uncontrolled cell division. Given the restructuring, the oncologist may apply a medicine that will be directed against the activated product of the ALK gene (Chrysotinib).

In situ Fluorescence Hybridization (FISH).
Microsatellite analysis shows the degree of violation of the DNA repair system, and immunohistochemistry - protein biomarkers located on the surface, in the cytoplasm and nuclei of tumor cells.
All these studies are part of the new product of the biomedical holding Atlas, the Solo test. Using this test, the oncologist receives information about the molecular profile of the tumor and how it affects the potential efficacy of a wide range of anticancer drugs.
Solo specialists are researching up to 450 genes and biomarkers to assess how a tumor can respond to the use of more targeted drugs for the treatment of cancer. For some of them, biomarker analysis is dictated by the manufacturer. For others, they use data from clinical studies and recommendations from the international community of oncologists.
In addition to the choice of targets for targeted therapy, molecular profiling helps to detect mutations that, on the contrary, make the tumor resistant to a specific treatment, or genetic features that are associated with increased toxicity and require individual dose adjustment.
For research, biopsy material or paraffinized blocks of postoperative material are used.
Molecular profiling provides additional information about the disease, but it is not always applicable to the choice of treatment. For example, in situations where standard therapy is sufficiently effective or surgical treatment is indicated. Clinical situations can be identified when such a study may be most useful:
- Rare type of tumor;
- Tumors with an unspecified primary focus (it is unknown where the tumor originally appeared, which gave metastasis);
- Those cases when the choice from several options of application of target therapy is required;
- The possibilities of standard therapy have been exhausted, and experimental treatment or the inclusion of a patient in clinical studies is required.
Solo project specialists advise oncologists or patients and suggest whether a test is needed in this case.
The result of the diagnosis also includes recommendations for clinical studies with a suitable test drug. The patient has the opportunity to take part in them.
Precision Medicine and Clinical Research
Usually in medical practice, general strategies are used to treat patients with a definite diagnosis. For small cell lung cancer, one strategy is used, for non-small cell, another. For cancer, this method is not always suitable. Due to differences at the molecular level, even with the same type of tumor, patients can receive ineffective or unnecessary treatment.
With the increase in research and the invention of targeted drugs, the approach to the treatment of cancer has begun to change. To increase the relapse-free period and the patient's life expectancy, the molecular profile of the tumor, the body's response to drugs and chemotherapy (pharmacogenomics) must be taken into account, and the main biomarkers must be known.

Precision medicine can significantly improve the prognosis of a particular patient, avoid serious side effects of cancer drugs and significantly improve the quality of life of the patient. But this method has drawbacks.
Targeted drugs are becoming more and more, and they have two main limitations: most molecular-targeting agents provide only partial suppression of signaling pathways and many of them are too toxic to be used in combination.
Imagine that you are an architect in Moscow. You face a difficult task - to solve the problem of traffic jams at rush hour by building one bridge. Molecular mechanisms can be compared with the movement of machines, and the bridge - the main drug, which should solve the main problem. It seems that several drugs (a series of bridges) aimed at major molecular disorders can solve this problem. But the toxicity of drugs increases and may be unpredictable.
We began to better understand the molecular processes of malignant tumors, but current methods of introducing accurate oncology into clinical practice are lagging far behind. To speed up the study of targeted therapy, scientists have developed two new approaches - Basket and Umbrella.

The essence of the Basket method is that patients with a specific biomarker are selected for the study, regardless of the location and name of the tumor. In May 2017, the FDA approved such a treatment for a biomarker called high microsatellite instability (MSI-H) or a defect in inconsistency recovery (dMMR).
Molecular disorders differ not only in different patients, but also within the same tumor. Heterogeneity is a big problem in oncology, to solve which the design of the study Umbrella was developed. For the Umbrella method, patients are first selected according to the type of malignant neoplasms, and then genetic mutations are taken into account.
Such studies help not only to collect information on the action of targeted drugs - sometimes this is the only way for patients who do not respond to standard treatment with registered drugs.
Clinical example
We decided to give an illustrative example of how the use of extended molecular profiling might look like.
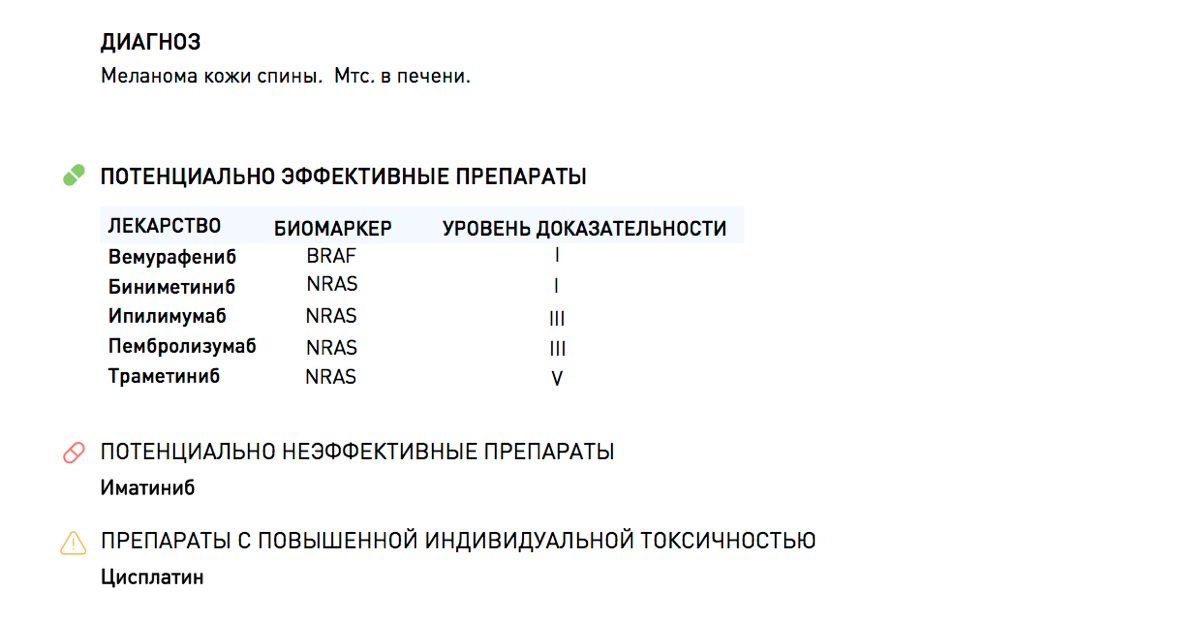
A patient with melanoma of the skin and metastases in the liver turned to an oncologist. The doctor and patient decided to do molecular profiling to get more complete information about the disease. The patient underwent a biopsy and sent tissue samples for examination. As a result of diagnosis, several important genetic disorders were found in the tumor:
- Mutation in the BRAF gene. Indicates the activation of the RAS-RAF-MEK oncogene signaling pathway, which is involved in cell differentiation and survival.
- Mutation in the NRAS gene. Indicates additional activation of the signaling cascade RAS-RAF-MEK.
- Hereditary variant of the TPMT gene. Indicates the characteristics of the metabolism of the anticancer drug "Cisplatin".

Focusing on the results of clinical studies and recommendations we can come to the following conclusions:
- Potentially effective drugs of the class of BRAF inhibitors (Vemurafenib) can be, moreover, the presence of the NRAS mutation may serve as an additional reason for the appointment of a double blockade of the signaling cascade - a combination with MEK inhibitors (Trametinib).
- Despite the fact that there is no approved therapy aimed directly at the NRAS oncogene, it is known that mutations in it increase the likelihood of successful treatment in the administration of immunotherapy (Ipilimumab and Pembrolizumab).
- The hereditary genetic variant in the TPMT gene indicates increased individual toxicity of Cisplatin, which requires dose adjustment when prescribing platinum-containing treatment regimens.
Thus, the doctor gets the opportunity to navigate among the possible treatment options based on not only the clinical parameters of the patient, but also taking into account the molecular characteristics of the tumor.
Molecular diagnosis is not a panacea for all oncological diseases. But it is an important tool for the oncologist, which allows you to approach the treatment of malignant tumors from the new side.
Thank you for reading and commenting on our oncology materials. Here is the full list of articles:
Source: https://habr.com/ru/post/370865/
All Articles