Stem cells can successfully replace dead brain cells

Researchers at the University of Munich Ludwig-Maximilian , Max Planck Institute for Neuroscience , as well as the Research Center. Helmholtz in Munich demonstrated that in mice, transplanted neurons derived from embryonic cells can actually be included in the existing network and correctly perform the tasks for which the damaged cells were responsible. This work is of great importance in the potential treatment of all acquired brain diseases, including stroke, trauma, neurodegenerative diseases such as Alzheimer's or Parkinson's. Each of these ailments leads to irreversible loss of nerve cells and lifelong neurological deficit.
When it comes to recovery, for example, after a stroke, the adult brain has very few opportunities to compensate for lost nerve cells. Therefore, doctors and researchers in the field of biomedicine are exploring the possibility of using transplanted nerve cells to replace neurons that have suffered as a result of injury or illness. Previous studies have shown that it is possible to eliminate at least some clinical symptoms by transplanting fetal nerve cells into damaged neural networks. However, the process of obtaining fetal cells raises many ethical issues, since this biomaterial can only be obtained from the fetus after an abortion, at about 9-12 weeks gestation. In addition, it is not completely clear whether the transplanted intact neurons can integrate well enough to restore the functions of the neural network.
')
In a study published in Nature on October 26, scientists found out whether embryonic nerve cells can be transplanted so that they successfully integrate into the neural network and function in the visual cortex of adult mice. “We know so much about the functions of nerve cells in this area that we can easily assess whether implanted cells actually perform the tasks that are assigned to the neural network,” said Professor Mark Hübener, one of the study leaders. Hübener specializes in the structure and functions of the mammalian visual system.
In their experiments, a team of researchers transplanted embryonic cells into the affected areas of the visual zone of adult mice. Over the following months, they observed the behavior of implanted immature neurons using two-photon microscopy . This method helped to find out whether they differentiate into so-called “ pyramidal cells ” - excitatory neurons. The fact that the cells survived and continued to develop is very encouraging. Everything became more interesting when researchers more closely studied the electrical activity of the transplanted cells. Joint research postgraduate Suzanne Faulkner and
A postdoc Sophia Grade showed that new cells formed synaptic connections that are usually established between cells of the network and responded to visual stimuli.
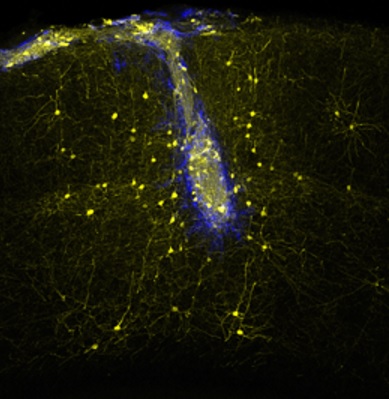
The image shows how nerve transplants (blue) are associated with intact areas of the network (yellow)
The team then proceeded to study the established connections after neuron transplantation. They found that pyramidal cells derived from transplanted mature neurons formed functional connections with the corresponding nerve cells throughout the brain. In other words, they successfully took the place of their predecessors. In addition, they were able to process the incoming information and transmit it further across the network. “These results show that implanted nerve cells with extreme precision united into a neural network, in which, under normal conditions, new nerve cells would never join,” explains Professor Magdalena Goetz, whose job was to find ways to replace lost neurons in the central nervous system. system. The new study shows that immature neurons are able to respond correctly to signals in the brain of adult mammals and can close functional gaps in the existing neural network.
One of the main advantages of transplantation of embryonic stem cells is that they do not produce tissue compatibility antigens. When the sets of antigens in the donor and the recipient do not match, this leads to cell rejection. But in the case of ESC transplantation this does not happen, and the chance that the cells will take root is usually very large. However, scientists have found that there are mechanisms that can prevent engraftment of new cells.
The process of creating neurons from stem cells and progenitor cells is called " neurogenesis ." Scientists have shown that epigenetic mechanisms that come into play in the early stages of neurogenesis have a significant influence on the further fate of neuronal neurons. These mechanisms affect inherited changes in the phenotype or expression of genes , but do not lead to a change in the sequence in DNA. To find out what significance early epigenetic modifications have on the development of nerve cells during embryogenesis in mice, Magdalina Goetz and her colleagues purposefully blocked the activity of the UHRF1 gene. The gene controls many epigenetic functions, including DNA methylation , by modifying the DNA molecule without affecting the nucleotide sequence. Methylation of certain nucleotide bases in DNA often serves to “turn off” certain genes.
The blocking of UHRF1 in the anterior region of the rat brain resulted in the activation of retroviral elements in the genome, which had previously been inhibited by methylation. Activation caused the accumulation of retroviral proteins in the affected cells and the deregulation of genes. This, in turn, led to progressive disturbances in vital cellular processes and accelerated mass cell death. The results of this study showed that even such factors, which act only at the very beginning of neurogenesis, can influence the fate of cells, which can manifest itself only weeks later.
Source: https://habr.com/ru/post/369829/
All Articles