How stable is the substance we are made of?
I believe that nature has stable laws of beauty and utility. Spring plants, autumn collects, and so on until the end of time.
- Robert Browning
Like everything else that exists in the universe - galaxies, stars, planets - people are composed of atoms.
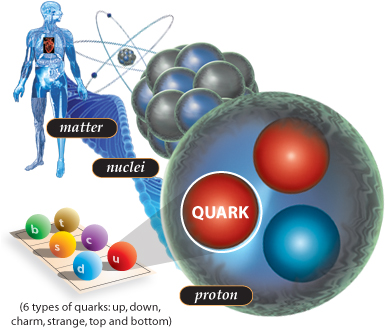
And just like galaxies, stars and planets, more than 99.9% of the mass of your body consists not just of atoms, but of atomic nuclei.
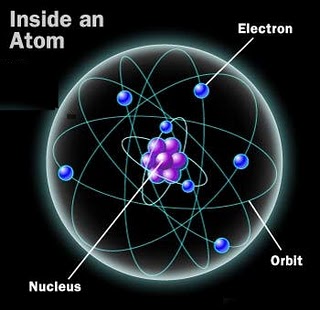
And if we climb into the very heart of the atom, we find that its nucleus consists of a combination of two simple nucleons: a proton and a neutron. Proton and neutron are connected in hundreds of different combinations, and determine not only the type of atom, but also its stability. And the human body is at least 10 28 atoms.
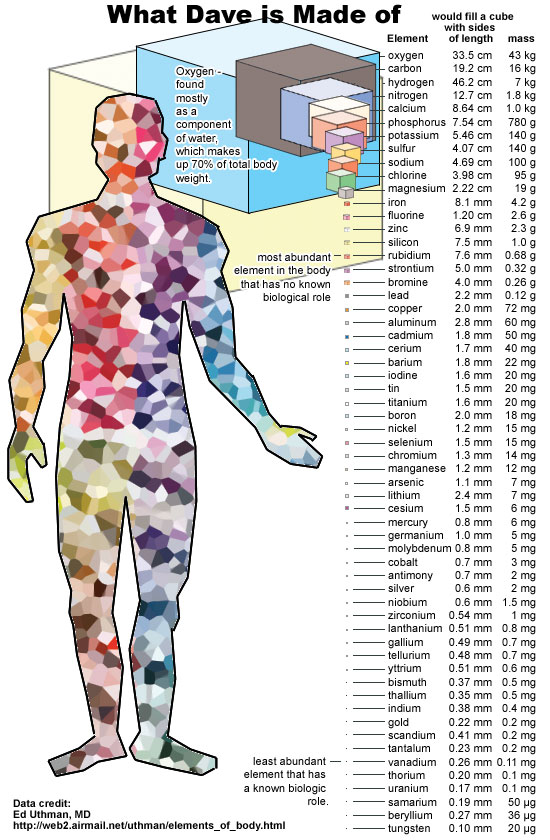
More than 10,000,000,000,000,000,000,000,000 atoms in each person. And some of these atoms are known for their radioactivity, for example, bismuth, uranium, thorium, but they always retain the total number of nucleons. Even a free neutron, although not stable, decays into a proton (and something else), keeping the total number of nucleons.
')
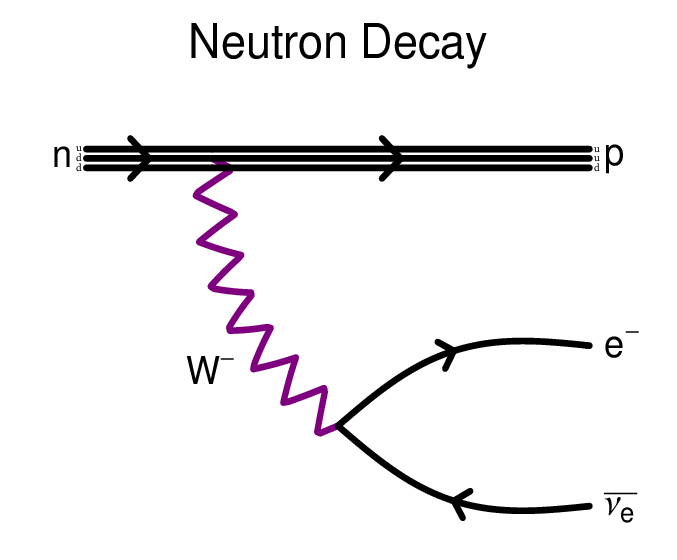
What about protons? More than 10 27 atoms in man are hydrogen atoms, in which the nucleus consists of only one proton. Is it possible that these protons are unstable? According to some ideas of physics (such, for example, as various theories of great unification), the proton can also decay!
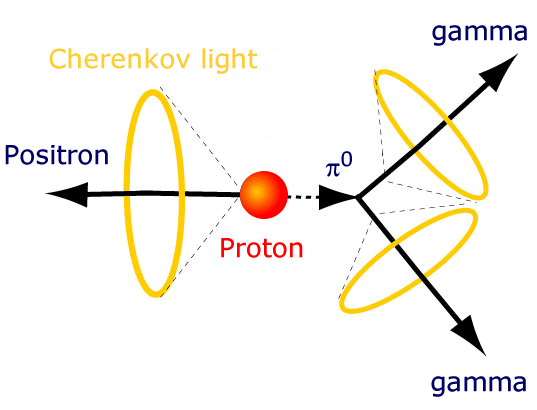
But if so, his lifetime should be very long. The neutron decays within 15 minutes, but the proton must live for a very long time. We can figure it out just by using our bodies. With 4 * 10 27 protons inside us - in the nuclei of hydrogen atoms - we cannot allow a large number of them to decay. Then we ourselves would let out too much energy!
You wonder why?
Just as matter is transformed into energy in the sun and atomic bombs, it could also come from such quiet things as protons. And you know what? After all, people actually radiate energy, like all warm-blooded primates.

In the visible spectrum, this is not obvious, but if you look in infrared light, you will see that people, relative to their environment, constantly radiate heat to the colder air around them.

To withstand the desired temperature, it is necessary to consume energy to compensate for the one that is constantly emitted. An adult of my size should emit 100 watts of energy — that's 100 joules per second, much like an incandescent lamp.

Even if you received 100% of this energy from decaying protons, this limits the number of protons that can decay every second to only 600 billion.
But based on the huge number of protons in the body, it can be understood that on average, a proton takes at least hundreds of millions of years to decay. And in reality, we do not emit 100 watts of energy due to proton decay.

We get it in the form of chemical energy by eating
But in order to accurately verify whether protons decay, it is clear what needs to be done. We need to collect them as much as possible in one place, build a giant detector and look for the signature of the decay.
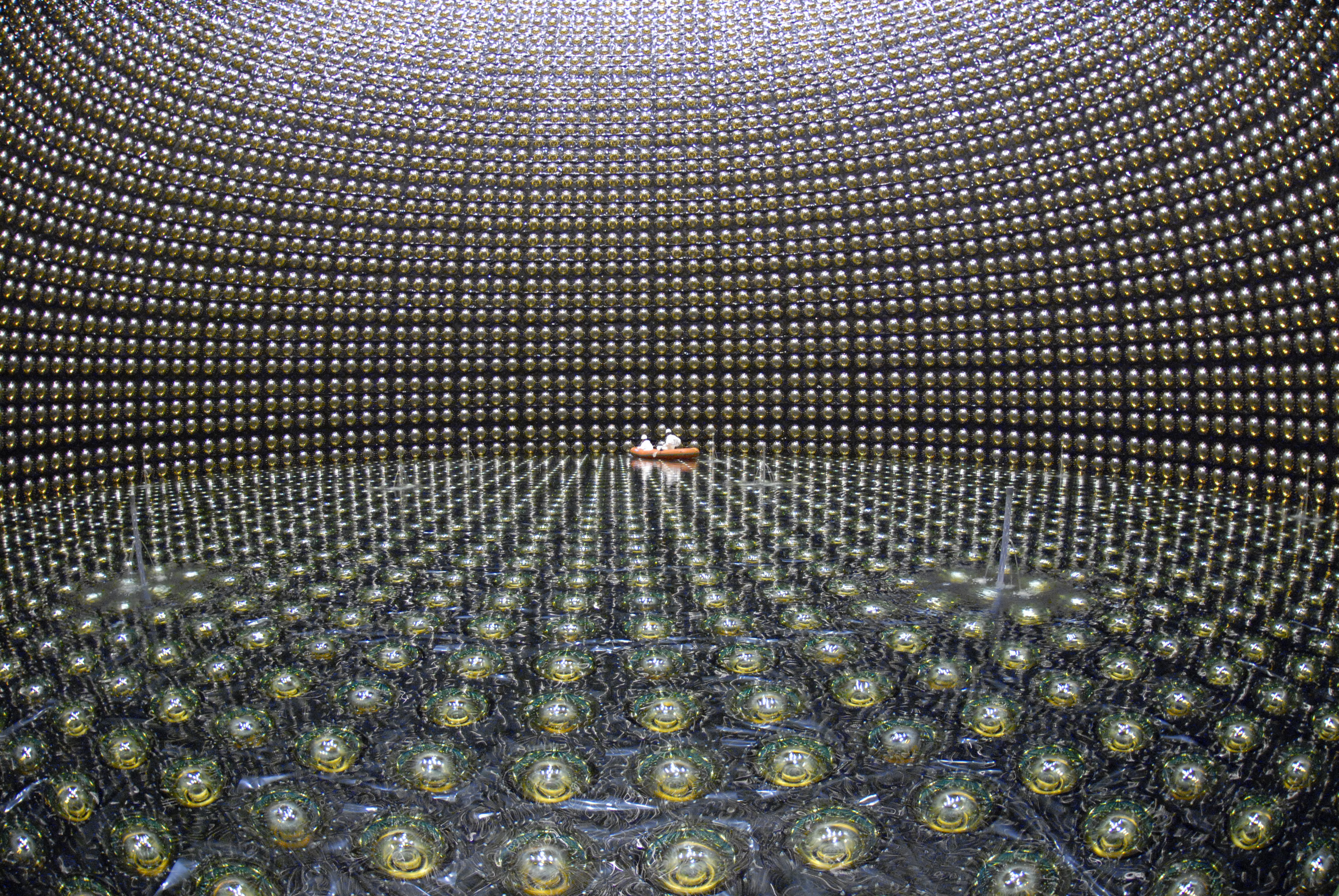
So they did in Kamioka, Japan. They built a reservoir with hundreds of tons of water and with photon detectors around the perimeter. If any of the protons decays, the high-energy decay products will leave light signals, allowing us to measure not only the fact of decay, but also the number of decaying atoms.
If you take a reservoir with 10 32 protons, wait a year, and find out that none of them broke up - it becomes clear that their half-life is at least 10 32 years!
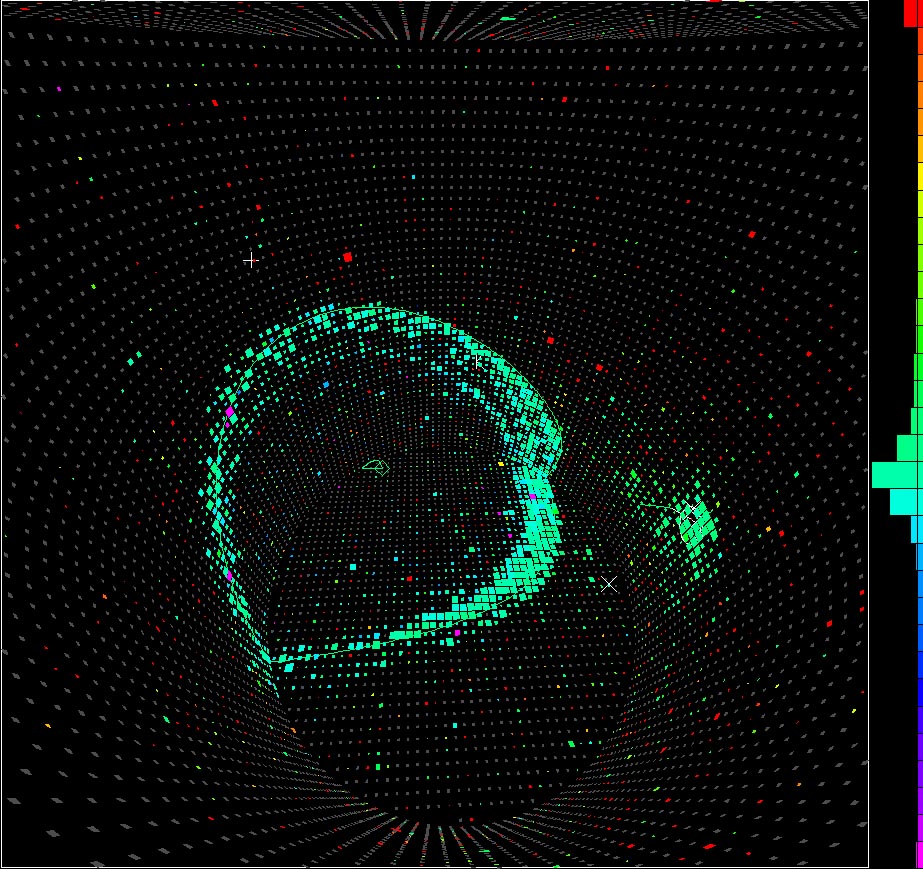
And although such a device turned out to be very useful for creating cosmic neutrino detectors, all experiments with proton decay yielded no results. In general, based on the data obtained, the proton lifetime should be at least 10 35 years, which is quite good, considering that the age of the Universe is only about 10 10 years!
Proton is so stable that it poses a problem for many Great Unification Theories. Only on the basis of the lifetime we have obtained, can we say that the chance that during your life any of the protons of your body will decay is 0.001%. And how stable is that matter at the fundamental level that we are made of?
Source: https://habr.com/ru/post/369725/
All Articles