The first direct proof of stable carbine - the most durable material in the world
Theoretically, carbin is 40 times stronger than diamond
 Carbon is very diverse in its modifications. Many carbon allotropes are known that possess unique properties: diamond, graphene, fullerene, etc., in total about a dozen allotropes. But in all this diversity there was one exception - carbin. This allotropic form consists of carbon fragments with a ternary –C≡C – bond.
Carbon is very diverse in its modifications. Many carbon allotropes are known that possess unique properties: diamond, graphene, fullerene, etc., in total about a dozen allotropes. But in all this diversity there was one exception - carbin. This allotropic form consists of carbon fragments with a ternary –C≡C – bond.Carbin could not be synthesized, although the properties of this material have been studied for a very long time. The reason for the failure is that the carbin is extremely unstable.
Failing after failure, the scientists reasoned that certain mechanical properties of carbyne should exceed those of all known carbon allotropes. It was assumed that its mechanical stiffness is 2 times greater than that of graphene; strength - 40 times more than diamond; tensile strength is also greater than that of any form of carbon. Well, other scientists believed that a stable form of carbyne does not exist at all.
')
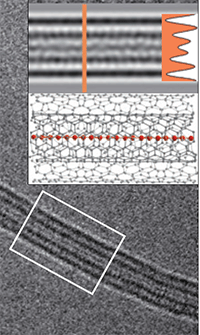
Now in this dispute put an end. We see the superlong 1D carbine molecule directly in the photograph (centered on the nanotube).
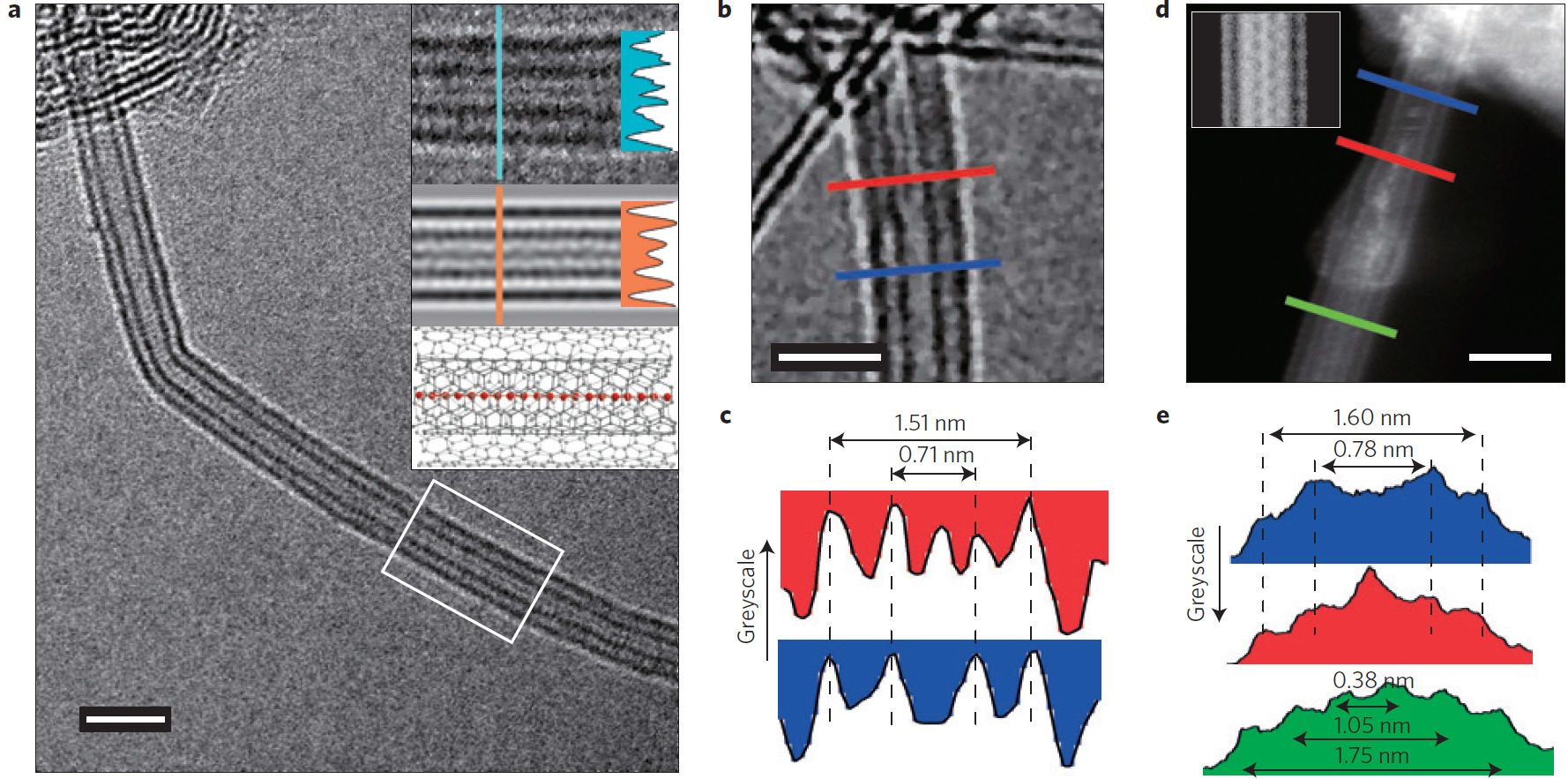
An international group of scientists for the first time found a method for mass production of stable carbyne. By "mass production" is meant the compilation of chains of atoms of great length that remain stable.
For the production of the material, they took two layers of graphene, squeezed them together and twisted them into thin carbon nanotubes with a double wall. These nanotubes surround the 1D carbine molecule and protect it from imminent disintegration.
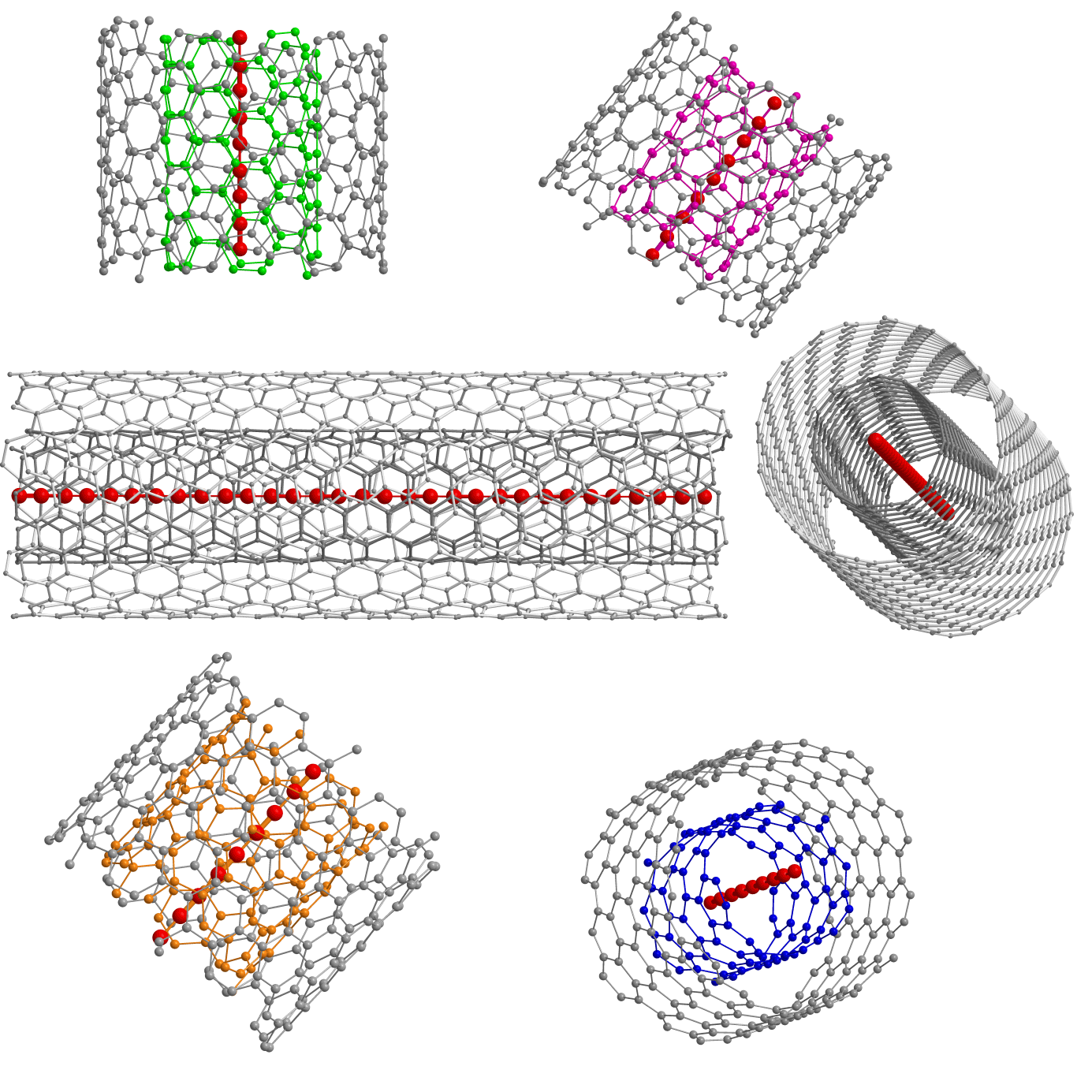
Until today, the maximum number of carbon atoms in a single continuous chain was 100 (2003). Now the record is broken, and very significantly: the new method allowed us to make a chain of 6400 atoms, and this is not the limit.
In addition, with the elongation of the chain, the electrical properties of carbyne improved. This means that scientists will receive material for interesting experiments.
The article "Closed linear carbon chains as the way to mass production of carbyne" was published in the journal Nature Materials (doi: 10.1038 / nmat4617; pdf ).
It is nice that in the published article there are several references to the works of domestic scientists, including an article in the journal Nature from 1969, where Soviet physicists Sladkov and Kudryavtsev described the properties of diamond, graphite and carbyne as allotropic forms of carbon.
It should be noted that the Nobel Prizes in Physics have recently been awarded for fundamental work on other allotropic forms of carbon: fullerene (1996) and graphene (2010), so the synthesis of carbyne is a valuable task for scientists.
Source: https://habr.com/ru/post/368711/
All Articles